Abstract
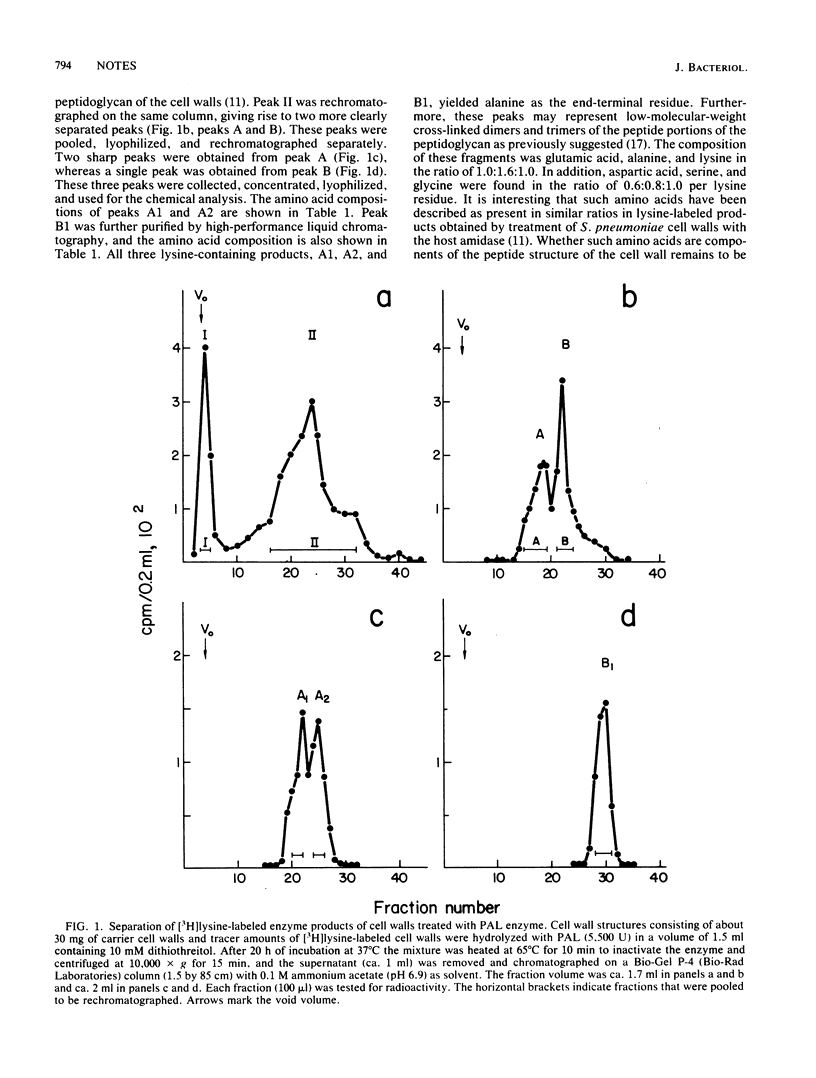
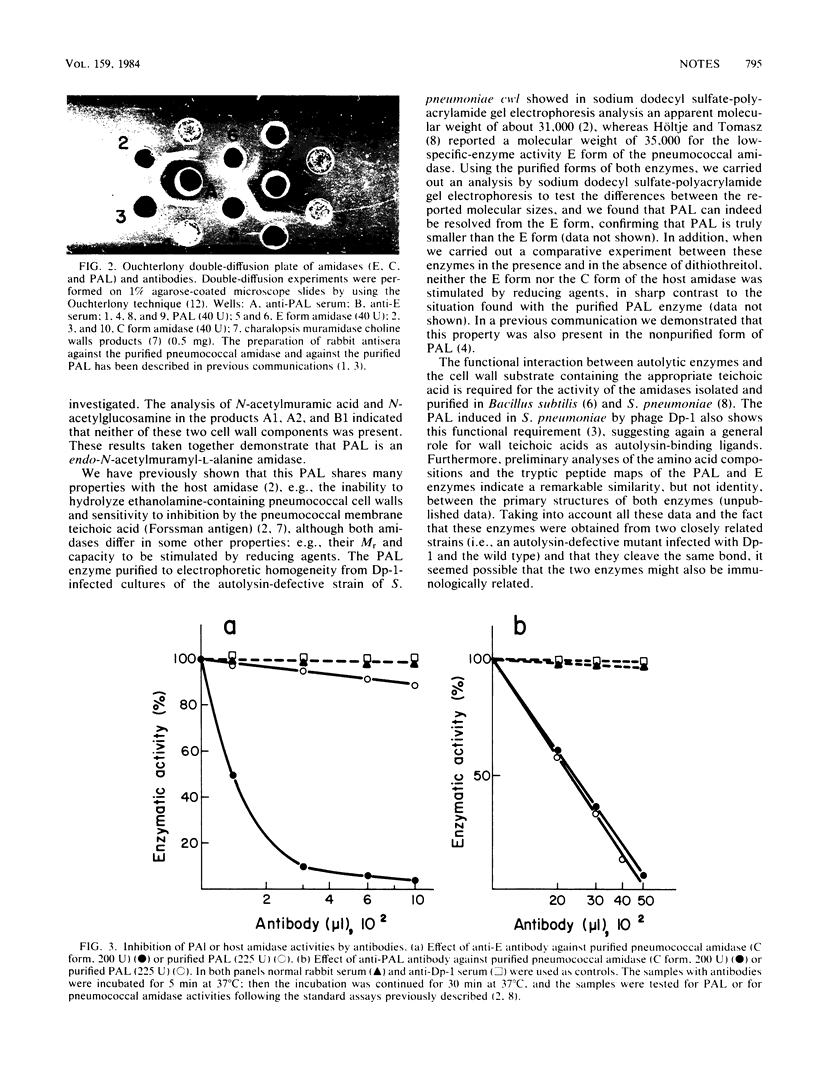
A phage-associated lysin recently isolated and purified from Streptococcus pneumoniae infected with bacteriophage Dp-1 has been biochemically characterized as an endo-N-acetyl-muramyl-L-alanine amidase. The purified peptides obtained after treatment of the cell wall with phage-associated lysin are composed of glutamic acid, alanine, lysine, glycine, serine, and aspartic acid in the molar ratios of 1.0:1.6:1.0:1.0:0.8:0.6. The N-terminal amino acid of this peptide has been characterized as alanine. This amidase and the inactive form of the amidase (E form) previously purified (J.V. Höltje and A. Tomasz, J. Biol. Chem. 251:4199-4207, 1976) from S. pneumoniae differ in their molecular weights, as well as in their capacity to be stimulated by reducing agents, and do not cross-react immunologically, although anti-phage-associated lysin serum was able to recognize and inhibit both phage-associated lysin and the active form (C form) of the host amidase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Garcia P., Garcia E., Ronda C., Lopez R., Tomasz A. A phage-associated murein hydrolase in Streptococcus pneumoniae infected with bacteriophage Dp-1. J Gen Microbiol. 1983 Feb;129(2):489–497. doi: 10.1099/00221287-129-2-489. [DOI] [PubMed] [Google Scholar]
- Garcia P., Lopez R., Ronda C., Garcia E., Tomasz A. Mechanism of phage-induced lysis in pneumococci. J Gen Microbiol. 1983 Feb;129(2):479–487. doi: 10.1099/00221287-129-2-479. [DOI] [PubMed] [Google Scholar]
- Herbold D. R., Glaser L. Interaction of N-acetylmuramic acid L-alanine amidase with cell wall polymers. J Biol Chem. 1975 Sep 25;250(18):7231–7238. [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Purification of the pneumococcal N-acetylmuramyl-L-alanine amidase to biochemical homogeneity. J Biol Chem. 1976 Jul 25;251(14):4199–4207. [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Specific recognition of choline residues in the cell wall teichoic acid by the N-acetylmuramyl-L-alanine amidase of Pneumococcus. J Biol Chem. 1975 Aug 10;250(15):6072–6076. [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez R., Ronda C., Tomasz A., Portoles A. Properties of "diplophage": a lipid-containing bacteriophage. J Virol. 1977 Oct;24(1):201–210. doi: 10.1128/jvi.24.1.201-210.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- Ronda-Lain C., Lopez R., Tapia A., Tomasz A. Role of the pneumococcal autolysin (murein hydrolase) in the release of progeny bacteriophage and in the bacteriophage-induced lysis of the host cells. J Virol. 1977 Jan;21(1):366–374. doi: 10.1128/jvi.21.1.366-374.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Cellular metabolism in genetic transformation of pneumococci: requirement for protein synthesis during induction of competence. J Bacteriol. 1970 Mar;101(3):860–871. doi: 10.1128/jb.101.3.860-871.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Model for the mechanism controlling the expression of competent state in Pneumococcus cultures. J Bacteriol. 1966 Mar;91(3):1050–1061. doi: 10.1128/jb.91.3.1050-1061.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Surface components of Streptococcus pneumoniae. Rev Infect Dis. 1981 Mar-Apr;3(2):190–211. doi: 10.1093/clinids/3.2.190. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Westphal M., Briles E. B., Fletcher P. On the physiological functions of teichoic acids. J Supramol Struct. 1975;3(1):1–16. doi: 10.1002/jss.400030102. [DOI] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]



