Abstract
Multidomain proteins account for over two-thirds of the eukaryotic genome. Although there have been extensive studies into the biophysical properties of isolated domains, few have investigated how the domains interact. Spectrin is a well-characterized multidomain protein with domains linked in tandem array by contiguous helices. Several of these domains have been shown to be stabilized by their neighbors. Until now, this stabilization has been attributed to specific interactions between the natural neighbors, however we have recently observed that nonnatural neighboring domains can also induce a significant amount of stabilization. Here we investigate this nonnative stabilizing effect. We created spectrin-titin domain pairs of both spectrin R16 and R17 with a single titin I27 domain at either the N- or the C-terminus and found that spectrin domains are significantly stabilized, through slowed unfolding, by nonnative interactions at the C-terminus only. Of particular importance, we show that specific interactions between natural folded neighbors at either terminus confer even greater stability by additionally increasing the folding rate constants. We demonstrate that it is possible to distinguish between natural stabilizing interactions and nonspecific stabilizing effects through examination of the kinetics of well chosen mutant proteins. This work adds to the complexity of studying multidomain proteins.
INTRODUCTION
Multidomain proteins account for between 60% and 80% of eukaryotic genomes (1–3). Although most studies have focused on investigations of the biophysical properties of individual domains (4), relatively few have looked at how these domains interact (3). In some proteins, domains behave in a cooperative manner, that is, the folding of one domain is influenced by its neighboring domains (5–12), whereas in others, domains fold independently (13–16).
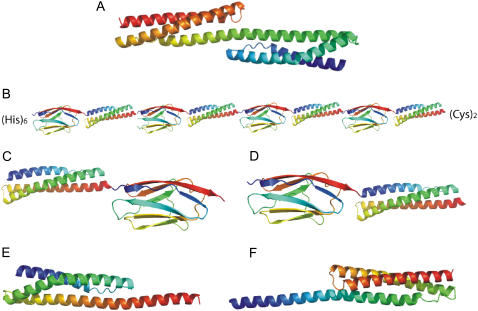
The multidomain protein system that has been studied in the most detail is the tandem repeat protein spectrin (5–7,17–19). Spectrin domains are three-helix-bundle, rodlike domains connected to each other by a contiguous helix that extends from one domain to the next (Fig. 1 A). These domains are both stabilized by their neighbors, as manifested by both a decrease in unfolding rate constants and, in some situations, an increase in refolding rate constants. This cooperativity has been attributed to the presence of the linking helix (5,17,19).
FIGURE 1.
Structures of the constructs used in this study. Each domain is colored from blue at the N-terminus to red at the C-terminus. (A) R1617. (B) R164I274. (C) R16-I27. (D) I27-R16. (E) R1617A. (F) 16CR17. (R174I274, R17-I27, and I27-R17 are not shown, as they have approximately the same structure as B, C, and D, respectively). The spectrin-titin domain pairs are separated by a two-residue linker from the incorporation of restriction sites between the domains (see Materials and Methods). The amino acid sequences of R16-(Pro)3-R17, R1617A, and 16CR17 are given in (5). In B, the (His)6 tag is required for purification and the (Cys)2 enables anchoring of the protein to a gold surface for atomic force microscopy measurements.
The 16th and 17th domains of chicken brain α-spectrin (R16 and R17) have been investigated in detail. In isolation, both domains are stable and folded in solution and unfold in a cooperative two-state manner (18). The tandem pair, R1617, is thermodynamically more stable than either domain alone (5,17) and it has been shown, through equilibrium studies and kinetic analysis, to behave as a cooperative unit. At equilibrium, the tandem protein acts as a two-state system: only fully folded or fully unfolded species are populated; no intermediates (with one domain folded and one unfolded) are detectable. Similarly, except at very high denaturant concentrations, there is only one folding and one unfolding phase (6). Kinetic analysis has revealed that the folding mechanism proceeds as shown in Fig. 2. R16 folds first, followed by rapid folding of R17. Unfolding is the reverse process, with R17 unfolding first, and then R16. Thus, kinetics of R16 can only be determined in the presence of unfolded R17, whereas R17 kinetics are determined only in the presence of folded R16. Crucially kinetic analysis was essential to determine the stability of each domain in the two-domain pair. Equilibrium measurements alone are insufficient to allow determination of the overall stability of the protein (6,7). Both domains are stabilized by their neighbor. Unfolded R17 stabilizes the native, folded R16 domain by decreasing its unfolding rate constant. Conversely, the folded R16 domain stabilizes R17 by both decreasing the unfolding rate constant and increasing the rate constant of refolding (6).
FIGURE 2.
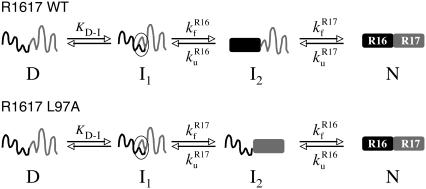
Comparison of the folding pathways of (top) R1617 WT and (bottom) R1617 L97A. I1 is a rapidly formed unstable intermediate that slows the folding rate and causes curvature in the folding arm of the chevron.
Throughout our previous analyses of stabilizing effects in this multidomain protein (and in the neighboring pair, R1516 (7)), we made the reasonable, and apparently universally held, assumption that the stabilizing interactions are specific interdomain interactions between the coevolved domains. However, we recently constructed artificial, multidomain “polyproteins” containing spectrin repeats to study the effect of force on spectrin domains in atomic force microscopy experiments (20). To prevent cooperative effects between the spectrin domains mediated by the helical linker, we separated the domains by the stable, mechanically strong all-β-sheet protein titin I27 (the 27th Ig domain from the I-band of human cardiac titin) (Fig. 1 B). Surprisingly, we found that both R16 and R17 were stabilized when flanked by I27 domains. Furthermore, the stabilization of R16 was of the same magnitude as observed in R1617 (Fig. 3).
FIGURE 3.
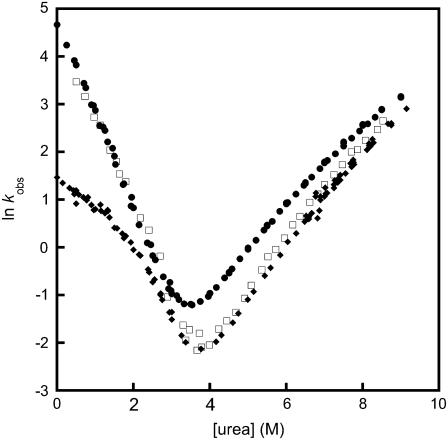
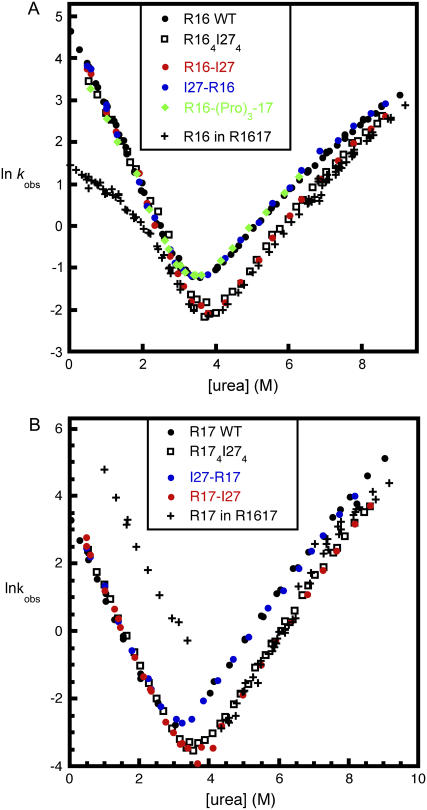
Comparison of the observed folding and unfolding rate constants of R16 rate alone, in the artificial multimeric protein R164I274, and in R1617. R16 WT (circles); R16 domain in R1617 (diamonds); R16 in R164I274 (squares). Rollover in the folding arm of R16 in R1617 is due to the rapid formation of an unstable intermediate (I1 in Fig. 2). Taking this into account, the folding rate constants in all constructs are the same. The unfolding rate constant is lower in R1617 and R164R174. Data for R16 WT and R17 WT are taken from Scott et al. (18), and data for R1617 from Batey et al. (6).
Here, we summarize an investigation into this nonnative stabilizing effect. We created spectrin-titin domain pairs of R16 and R17 each coupled with a single I27 domain at either the N- or the C-terminus. Fig. 1 shows cartoons of all the proteins used in this study. Our results demonstrate that spectrin domains can be significantly stabilized by nonnative interactions at the C-terminus, whereas they are unaffected by nonnative interactions at the N-terminus. This effect arises from stabilization of the native state, resulting in lowering of unfolding rate constants. However, native interactions between folded domains confer even greater stability through increasing refolding rate constants and further lowering of the unfolding rate constants.
MATERIALS AND METHODS
Protein expression and purification
The choice of domain boundaries for R16 and R17 has previously been discussed (17,18) and the biophysical properties and folding of both domains in solution have been reported (18,21,22). The R1617 construct was designed to include all residues of the individual domains and has been described in detail elsewhere (5). Expression and purification of extensions of the single R16 and R17 domains with the adjoining helix from the adjacent domain (R1617A and 16CR17) has also been described previously (5). This study also describes the cloning and expression of a mutant of R1617 where the domains are separated by three proline residues (R16-(Pro)3-R17). Cloning, expression, and biophysical characterization of I27 has been described previously (23,24). The multimeric construct R164I274 was made using a modified version of an existing versatile cloning system (25) and is described in more detail elsewhere (20).
Four pairs of spectrin-titin domains (R16-I27, I27-R16, R17-I27, and I27-R17) were constructed for this study using standard molecular biology techniques. BamHI restriction sites were introduced at the N-terminus of each domain pair and EcoRI at the C-terminus to allow cloning into a modified version of the pRSETA vector (Invitrogen, Carlsbad, CA), that contains a His6-tag for purification. Spectrin and titin domains were linked using the restriction sites of SacI (I27-R16, I27-R17) or BssHII (R16-I27, R17-I27), which leave a two-residue linker (Glu-Leu and Ala-Arg, respectively) between the domains.
All protein expression was carried out in Escherichia coli C41 cells (26) according to the protocol described previously (18). Since all proteins were cloned into a modified version of the pRSETA vector (Invitrogen), which contains a His6-tag, initial purification used affinity chromatography using standard methods on a Ni2+-agarose resin. The proteins were then purified further using size-exclusion chromatography on an AKTA FPLC (Amersham, Princeton, NJ). Purified proteins were dialyzed into 50 mM sodium phosphate buffer (pH 7.0) and used immediately.
Stability measurements
The stability of the spectrin-titin proteins was determined by equilibrium denaturation using urea as the denaturant. Sixty-eight solutions at different concentrations of urea were prepared, each with a final protein concentration of 1 μM in 5 mM dithiothreitol and left for at least 3 h to equilibrate. Experiments were performed on a Perkin Elmer (Wellesley, MA) fluorescence spectrometer. Proteins were excited at a wavelength of 280 ± 2.5 nm, with the emission being monitored at 350 ± 2.5 nm. All experiments were carried out at 25 ± 0.1°C in 50 mM sodium phosphate buffer (pH 7.0). The data were fit to a two-state transition, as described elsewhere (27,28).
Kinetic measurements
Kinetic experiments were carried out using Applied Photophysics SX.18MV and SX.20MV stopped-flow fluorimeters at a temperature of 25 ± 0.1°C in 50 mM sodium phosphate buffer (pH 7.0). A final concentration of 1 μM protein in 5 mM dithiothreitol was used. Between 6 and 10 traces were averaged for typical measurements. Proteins were excited at a wavelength of 280 nm, and a 335-nm cut-off filter was used for all proteins. For refolding kinetics, proteins were initially unfolded in urea at concentrations of ≤5 M. Below 5 M urea, I27 is folded and so all kinetic amplitude comes from the refolding of spectrin domains. Above this concentration, I27 begins to unfold and will contribute to the observed rate. Kinetics were fit to an equation describing either a single or a double exponential process.
RESULTS
I27 and spectrin domains have different stabilities
I27 and the spectrin domains have different stabilities and spectral properties that allow the stability and kinetics of the spectrin domains to be distinguished from those of I27. I27 unfolds an order of magnitude more slowly than either of the spectrin domains used in this study (18,24). Thus in unfolding studies, it is easy to separate the unfolding of the titin and the spectrin domains. I27 is also more stable and only begins to unfold above 6 M urea, where R16 and R17 are already unfolded. In refolding experiments, we always started with protein unfolded in urea solutions that were ≤5 M so we could be confident that the data obtained reported only the refolding of the spectrin domains.
R16 and R17 are stabilized by I27 at the C-terminus but not at the N-terminus
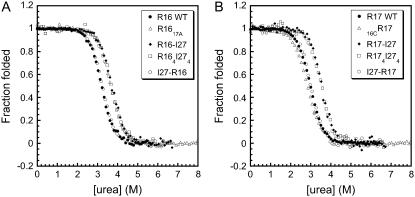
In the artificial polyprotein (Fig. 1 B), the spectrin domains are flanked by I27, i.e., most spectrin domains have I27 at both the N- and the C-terminus, except for the domain nearest the Cys2 motif, which has I27 only at the N-terminus. Four different two-domain proteins were therefore constructed here: two have I27 attached to the N-terminus of the spectrin domains (I27-R16 and I27-R17) and two have I27 attached to the C-terminus (R16-I27 and R17-I27) (Fig. 1, C and D). Equilibrium denaturation experiments showed that spectrin domains were stable and folded in solution in all constructs (Table 1). The unfolding profiles, displayed as the fraction of protein folded, for each construct is shown in Fig. 4. It is clear that the presence of I27 at the C-terminus stabilizes both R16 and R17 by ∼1 kcal mol−1 (Table 1). No stabilization of either spectrin domain is observed when I27 is the N-terminal domain.
TABLE 1.
Equilibrium properties of spectrin domains in the proteins discussed
| Protein | ΔGD-N (kcal mol−1) | ΔΔGD-N (kcal mol−1) |
|---|---|---|
| R16 domain | ||
| R16 WT | 6.1* | – |
| R16 in R1617 (R17 unfolded) | 6.9* | −0.8* |
| R16 in R164I274 | 6.9 | −0.8 |
| R16 in R16-I27 | 7.0 | −0.9 |
| R16 in I27-R16 | 6.1 | 0.0 |
| R16 in R1617A | 7.0* | −0.9 |
| R16 L97A | 2.4† | – |
| R16 in R1617 L97A (R17 folded) | 5.6‡ | −3.2 |
| R16 in R16-(Pro)3-R17 | 6.1‡ | 0.0 |
| R17 domain | ||
| R17 WT | 5.7* | – |
| R17 in R1617 (R16 folded) | 7.5* | −2.8* |
| R17 in R174I274 | 6.8 | −1.1 |
| R17 in R17-I27 | 6.7 | −1.0 |
| R17 in I27-R17 | 5.7 | 0.0 |
| 16CR17 | 5.4* | 0.4 |
| R17 in R1617 L97A (R16 unfolded) | 5.7‡ | 0.0 |
FIGURE 4.
Equilibrium denaturation of spectrin-titin domain pairs, multimeric proteins, and extension mutants compared to wild-type. (A) Proteins containing R16 domains. R16 in I27-R16 (open circles) has the same stability, within error, as R16 WT (solid circles), whereas the R16 domain in R164I274 (squares), R16-I27 (diamonds) and R1617A (triangles) are all stabilized (to the same extent) relative to WT. (B) Proteins containing R17 domains. R17 in I27-R17 (open circles) and 16CR17 (triangles) have the same stability, within error, as R17 WT (solid circles), whereas the R17 domain in both R174I274 (squares) and R17-I27 (diamonds) are stabilized (to the same extent) compared to WT. Data for R16 WT and R17 WT are taken from Scott et al. (18), and data for R1617 and the extension mutants from Batey et al. (5).
The stabilization of R16 and R17 by a C-terminal I27 is due to lowered unfolding rate constants
Studies of the folding kinetics of all four I27 constructs revealed that I27 extensions at either end of the spectrin domains had no effect on the folding rate constants of either R16 or R17 (Fig. 5); i.e., I27 was not affecting the relative stabilities of the transition states for folding. However, the unfolding rate constants of the spectrin domains in R16-I27 and R17-I27 were both lower than wild-type. This decrease in the unfolding rate constants accounted for the full increase in stability observed in the equilibrium experiments. This indicates that a C-terminal I27 causes stabilization solely of the native state of the spectrin domains.
FIGURE 5.
Kinetic chevron plots for spectrin-titin domain pairs compared to multimeric proteins and WT. (A) Proteins containing R16 domains. In all proteins, the folding rate constant of the R16 domain is the same, within error. R16 in I27-R16 (blue circles) unfolds at the same rate, within error, as R16 WT (black circles) and R16 in R16-(Pro)3-17 (green diamonds), whereas both the R16 domains in R164I274 (squares) and R16-I27 (red circles) have slower unfolding rate constants (which are the same, within error). This stabilization is of the same magnitude as induced by unfolded R17 in R1617 (crosses) (see also Table 1). (Curvature seen in the folding arm for R16 in R1617 is explained in the legends to Figs. 2 and 3.) (B) Proteins containing R17 domains. The R17 domain in I27-R17 (blue circles) unfolds at the same rate, within error, as R17 WT (black circles). The R17 domain in both R174I274 (squares) and R17-I27 (red circles) unfold more slowly than WT and unfold at the same rate as R17 when stabilized by R16 in R1617 (crosses). The rate constant for folding of R17 is only increased by the presence of a folded R16 domain in R1617. Data for R16 WT and R17 WT are taken from Scott et al. (18), and data for R1617 from Batey et al. (6).
Spectrin domains are stabilized at the C-terminus by more than six residues of the naturally occurring neighboring domain
Interestingly, the stabilization induced by folded I27 at the C-terminus of R16 is approximately the same as that induced by the presence of an unfolded R17. Both effects are caused by a decrease in the unfolding rate constants (Table 1 and Fig. 5 A). To determine what other additions to the C-terminus of spectrin domains increase their stability, we analyzed a number of other constructs.
R16 cannot be considered to be “too short”
One possibility is that the spectrin domain boundaries had been wrongly assigned. When isolated domains from multidomain proteins are cut “too short” they can be artificially destabilized (15,29,30). Domain boundaries for R16 and R17 used in this study (18) are one residue shorter at the N-terminus and six residues longer at the C-terminus than the NMR-defined boundaries (31). This phasing ensured that the boundaries were consistent with those in MacDonald and Pozharski (17). When the stability of our construct was compared to one with the original NMR-defined boundaries, it was found that the C-terminal extension had no effect (32), i.e., the R16 used in our experiments was as stable as a significantly shorter construct, suggesting that the R16 construct we used here is not too short.
Addition of an extra 19 residues at the C-terminus stabilizes R16
More recently (5), we demonstrated that adding the A-helix of R17 (a total of 19 residues) to the C-terminal end of R16 (R1617A) increased the stability of R16 by 0.8 kcal mol−1. This stability change is close to what we observed upon the addition of I27 or a complete unfolded R17 domain to R16 (Table 1).
Spectrin domains are stabilized by their natural neighbor more than by I27
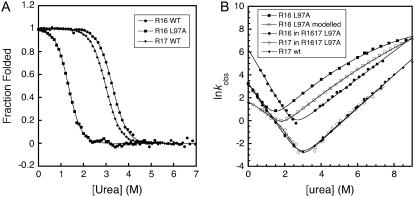
Although it is clear that spectrin domains are stabilized by any C-terminal extension of >6 six residues (i.e., by unfolded R17, by a fragment of R17, or by folded I27), it is important to establish whether the presence of the folded, natural R17 domain at the C-terminus of R16 has an additional stabilization effect. For wild-type R1617 this is not easy to determine, since the R16 domain in R1617 always unfolds after R17, so it is not possible to calculate the stabilizing effect folded R17 has on R16. However, by using a highly destabilizing mutation, L97A, in the R16 domain of R1617 it is possible to make R16 unfold first, so we can observe the folding of R16 in the presence of folded R17. This mutant destabilizes the isolated R16 domain by 3.7 kcal mol−1 (Fig. 6 and Table 1) (21).
FIGURE 6.
R16 is stabilized by folded R17. (A) equilibrium curves of R16 WT, R17 WT, and R16 with the mutation L97A. (B) Comparison of the kinetics of the R1617 L97A protein to its constituent domains. Kinetics of R16 with the mutation L97A (solid squares), R17 WT (diamonds), the R16 domain in R1617 L97A (solid circles) and the R17 domain in R1617 L97A (open circles). To show that the folded natural neighbor R17 slows the unfolding rate more than does a nonnatural neighbor, modeled data for the hypothetical R16 L97A-I27 protein are also shown (open squares). These data were simulated assuming a stabilization of the native state only by 0.95 kcal mol−1 (which causes a slowing of the unfolding rate, as is observed when I27 is positioned at the C-terminus of the spectrin domains (Table 1)). Data for R16 WT and R17 WT and R16 L97A are taken from Scott and co-workers (18,21).
In wild-type R1617, folding begins with formation of an intermediate that has structure around the connecting helix. This is then followed by folding of the R16 domain to result in a second intermediate with a folded R16 domain and an unfolded R17 domain. However, in R1617 L97A this folding pathway is altered. The first intermediate state is still formed, but the second step is the folding of the R17 domain, leading to an intermediate that has a folded R17 domain and an unfolded R16 domain. In both cases, the unfolding pathway is the reverse of the folding pathway (Fig. 2). It is important to note that in R1617 L97A, the R16 domain folds and unfolds in the presence of a folded R17 domain, allowing us to determine the stabilization effect of a natural, folded C-terminal domain.
The R17 rate constants from R1617 L97A overlay those of R17 alone, except at concentrations of denaturant where intermediate I1 is populated. This indicates that R17 is not stabilized by an unfolded domain at the N-terminus, in agreement with the fact that a nonnative N-terminal extension does not affect stability. R16 in R1617 L97A is significantly stabilized (3.2 kcal mol−1) by the folded, natural, R17 domain at the C-terminus through both a decrease in unfolding rate and an increase in folding rate. It is important to note that the unfolding rate is decreased by more than would be expected from either a folded nonnatural extension or from an unfolded natural extension (∼1 kcal mol−1). Also note that the addition of the natural folded R17 domain at the C-terminus significantly increases the refolding rate constant, which was not observed with I27 at this position. This reiterates that stabilization by an unfolded domain or a nonnatural folded domain is due purely to stabilization of the native state; the natural neighbor is the only one that also stabilizes the transition state for folding.
Insertion of helix-breaking residues prevents stabilization of R16
Helices, particularly those in peptides, are known to fray at the ends (17,33). By adding a fully folded I27 domain or even just another helical motif to the C-terminus, R16 is stabilized. It is possible, therefore, that in these constructs the structure of the C-helix of R16 is being maintained, albeit by nonnative interactions, and thus it is stabilized compared to the nontethered R16. If we prevent extra helical formation at the C-terminus of R16 by inserting proline residues between R16 and R17, R16 is no longer stabilized by unfolded R17. The folding and unfolding rate constants of R16 in R16-(Pro)3-R17 are the same as for wild-type R16 (R16 WT) (Fig. 5 A).
DISCUSSION
Several previous studies on multidomain protein systems have shown that stabilization of domains is achieved through the slowing of unfolding rates only (9,11). It has generally been assumed that this stabilization results from specific interactions between neighboring domains in the folded protein that stabilize the native state. However, we have shown here that this may not be the case, as, remarkably, both R16 and R17 are stabilized by the addition of a nonnatural neighbor, an all-β-sheet domain. This effect was only observed at the C-terminus, and only through extension well beyond the accepted domain boundaries. The observation that it is addition at only one terminus and not the other strongly suggests that the observed stabilization is not a simple consequence of molecular crowding (34). The addition of helix-breaking proline residues prevents stabilization, leading us to infer that any significant extension to the C-terminus reduces fraying of the C-helix of the spectrin domains, thus stabilizing the native fold.
It is of critical importance that natural interactions between folded domains stabilize the proteins to a significantly larger extent. R16 is stabilized by ∼1 kcal mol−1 by an unfolded R17 domain or an I27 domain, but is stabilized by 3.2 kcal mol−1 by folded R17 (as judged by the L97A mutant). This is similar to the stabilization of R17 by a folded R16 domain at the N-terminus (2.8 kcal mol−1, Table 1). Thus, we infer that native interactions between two folded spectrin domains stabilize the individual domains by >2 kcal mol−1 over stability induced by nonnatural extensions.
Addition of a partial natural domain (16CR17), an unfolded domain, or a folded, nonnatural domain at the N-terminus of the spectrin domains does not stabilize either spectrin domain (Table 1). However, addition of a folded domain at the N-terminus stabilizes R16 in R1516 (where the preceding domain R15 folds first (7)), and stabilizes R17 in R1617 where R16 folds first. It is of note that only the folded, natural extension to the domain has this effect on the folding kinetics. We see here that a folded I27 at the N-terminus has no effect on the rate of folding, whereas a natural, folded N-terminal extension can increase the folding rate constants considerably. R17 folds faster and is stabilized by folded R16, with the same effect being seen for R16 in R1516 (6,7). Thus, native interactions stabilize both the folded state and the partially folded transition state.
CONCLUSION
This work adds to the complexity of studying multidomain proteins. It may no longer be acceptable to assume that stabilization of a domain is simply due to specific interactions between the domain and its neighbor, since it may arise from nonspecific effects such as prevention of helix-fraying by lengthening the domain. However, we have shown that it is possible to distinguish between natural stabilizing interactions and nonspecific stabilizing effects by careful examination of the kinetics of well chosen mutant proteins. We find that in these spectrin domains, increases in the folding rate constants (and hence stabilization of the transition state for folding) come only through specific interactions between a natural folded domain, at either terminus, and its neighbor.
Acknowledgments
This work was supported by the Wellcome Trust (grant 064417/Z/01/A). J.C. is a Wellcome Trust senior research fellow. L.G.R. holds a Biotechnology and Biological Sciences Research Council research studentship.
Abbreviations used: R16, the 16th α-helical repeat from chicken brain α-spectrin; R17, the 17th α-helical repeat from chicken brain α-spectrin; R1617, the 16th and 17th α-helical repeats from chicken brain α-spectrin in a tandem construct; I27, the 27th Ig domain from the I-band of human cardiac titin.
Editor: Heinrich Roder.
References
- 1.Apic, G., J. Gough, and S. A. Teichmann. 2001. Domain combinations in archaeal, eubacterial and eukaryotic proteomes. J. Mol. Biol. 310:311–325. [DOI] [PubMed] [Google Scholar]
- 2.Ekman, D., A. K. Bjorklund, J. Frey-Skott, and A. Elofsson. 2005. Multi-domain proteins in the three kingdoms of life: orphan domains and other unassigned regions. J. Mol. Biol. 348:231–243. [DOI] [PubMed] [Google Scholar]
- 3.Han, J. H., S. Batey, A. A. Nickson, S. A. Teichmann, and J. Clarke. 2007. The folding and evolution of multidomain proteins. Nat. Rev. Mol. Cell Biol. 8:319–330. [DOI] [PubMed] [Google Scholar]
- 4.Jackson, S. E. 1998. How do small single-domain proteins fold? Fold. Des. 3:R81–R91. [DOI] [PubMed] [Google Scholar]
- 5.Batey, S., L. G. Randles, A. Steward, and J. Clarke. 2005. Cooperative folding in a multi-domain protein. J. Mol. Biol. 349:1045–1059. [DOI] [PubMed] [Google Scholar]
- 6.Batey, S., K. A. Scott, and J. Clarke. 2006. Complex folding kinetics of a multidomain protein. Biophys. J. 90:2120–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batey, S., and J. Clarke. 2006. Apparent cooperativity in the folding of multidomain proteins depends on the relative rates of folding of the constituent domains. Proc. Natl. Acad. Sci. USA. 103:18113–18118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osvath, S., G. Kohler, P. Zavodszky, and J. Fidy. 2005. Asymmetric effect of domain interactions on the kinetics of folding in yeast phosphoglycerate kinase. Protein Sci. 14:1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wenk, M., R. Jaenicke, and E. M. Mayr. 1998. Kinetic stabilisation of a modular protein by domain interactions. FEBS Lett. 438:127–130. [DOI] [PubMed] [Google Scholar]
- 10.Jager, M., P. Gehrig, and A. Pluckthun. 2001. The scFv fragment of the antibody hu4D5–8: evidence for early premature domain interaction in refolding. J. Mol. Biol. 305:1111–1129. [DOI] [PubMed] [Google Scholar]
- 11.Rothlisberger, D., A. Honegger, and A. Pluckthun. 2005. Domain interactions in the Fab fragment: a comparative evaluation of the single-chain Fv and Fab format engineered with variable domains of different stability. J. Mol. Biol. 347:773–789. [DOI] [PubMed] [Google Scholar]
- 12.Zhou, Z., H. Feng, H. Zhou, Y. Zhou, and Y. Bai. 2005. Design and folding of a multidomain protein. Biochemistry. 44:12107–12112. [DOI] [PubMed] [Google Scholar]
- 13.Politou, A. S., M. Gautel, S. Improta, L. Vangelista, and A. Pastore. 1996. The elastic I-band region of titin is assembled in a “modular” fashion by weakly interacting Ig-like domains. J. Mol. Biol. 255:604–616. [DOI] [PubMed] [Google Scholar]
- 14.Scott, K. A., A. Steward, S. B. Fowler, and J. Clarke. 2002. Titin; a multidomain protein that behaves as the sum of its parts. J. Mol. Biol. 315:819–829. [DOI] [PubMed] [Google Scholar]
- 15.Steward, A., S. Adhya, and J. Clarke. 2002. Sequence conservation in Ig-like domains: the role of highly conserved proline residues in the fibronectin type III superfamily. J. Mol. Biol. 318:935–940. [DOI] [PubMed] [Google Scholar]
- 16.Robertsson, J., K. Petzold, L. Lofvenberg, and L. Backman. 2005. Folding of spectrin's SH3 domain in the presence of spectrin repeats. Cell. Mol. Biol. Lett. 10:595–612. [PubMed] [Google Scholar]
- 17.MacDonald, R. I., and E. V. Pozharski. 2001. Free energies of urea and of thermal unfolding show that two tandem repeats of spectrin are thermodynamically more stable than a single repeat. Biochemistry. 40:3974–3984. [DOI] [PubMed] [Google Scholar]
- 18.Scott, K. A., S. Batey, K. A. Hooton, and J. Clarke. 2004. The folding of spectrin domains I: wild-type domains have the same stability but very different kinetic properties. J. Mol. Biol. 344:195–205. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald, R. I., and J. A. Cummings. 2004. Stabilities of folding of clustered, two-repeat fragments of spectrin reveal a potential hinge in the human erythroid spectrin tetramer. Proc. Natl. Acad. Sci. USA. 101:1502–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Randles, L. G., R. W. Rounsevell, and J. Clarke. 2007. Spectrin domains lose cooperativity in forced unfolding. Biophys. J. 92:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott, K. A., L. G. Randles, and J. Clarke. 2004. The folding of spectrin domains II: φ-value analysis of R16. J. Mol. Biol. 344:207–221. [DOI] [PubMed] [Google Scholar]
- 22.Scott, K. A., L. G. Randles, S. J. Moran, V. Daggett, and J. Clarke. 2006. The folding pathway of spectrin R17 from experiment and simulation: using experimentally validated MD simulations to characterize States hinted at by experiment. J. Mol. Biol. 359:159–173. [DOI] [PubMed] [Google Scholar]
- 23.Carrion-Vazquez, M., A. F. Oberhauser, S. B. Fowler, P. E. Marszalek, S. E. Broedel, J. Clarke, and J. M. Fernandez. 1999. Mechanical and chemical unfolding of a single protein: a comparison. Proc. Natl. Acad. Sci. USA. 96:3694–3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fowler, S. B., and J. Clarke. 2001. Mapping the folding pathway of an immunoglobulin domain: structural detail from φ-value analysis and movement of the transition state. Structure. 9:355–366. [DOI] [PubMed] [Google Scholar]
- 25.Steward, A., J. L. Toca-Herrera, and J. Clarke. 2002. Versatile cloning system for construction of multimeric proteins for use in atomic force microscopy. Protein Sci. 11:2179–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miroux, B., and J. E. Walker. 1996. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 260:289–298. [DOI] [PubMed] [Google Scholar]
- 27.Pace, C. N. 1986. Determination and analysis of urea and guanidine hydrochloride denaturation curves. Methods Enzymol. 131:266–280. [DOI] [PubMed] [Google Scholar]
- 28.Clarke, J., and A. R. Fersht. 1993. Engineered disulfide bonds as probes of the folding pathway of barnase: increasing the stability of proteins against the rate of denaturation. Biochemistry. 32:4322–4329. [DOI] [PubMed] [Google Scholar]
- 29.Politou, A. S., M. Gautel, C. Joseph, and A. Pastore. 1994. Immunoglobulin-type domains of titin are stabilized by amino-terminal extension. FEBS Lett. 352:27–31. [DOI] [PubMed] [Google Scholar]
- 30.Hamill, S. J., A. E. Meekhof, and J. Clarke. 1998. The effect of boundary selection on the stability and folding of the third fibronectin type III domain from human tenascin. Biochemistry. 37:8071–8079. [DOI] [PubMed] [Google Scholar]
- 31.Pascual, J., M. Pfuhl, D. Walther, M. Saraste, and M. Nilges. 1997. Solution structure of the spectrin repeat: a left-handed antipararallel triple-helical coiled-coil. J. Mol. Biol. 273:740–751. [DOI] [PubMed] [Google Scholar]
- 32.Scott, K. A. 2004. Biophysical studies of multidomain proteins. PhD thesis. University of Cambridge, Cambridge, UK.
- 33.Serrano, L., J. Sancho, M. Hirshberg, and A. R. Fersht. 1992. α-helix stability in proteins. I. Empirical correlations concerning substitution of side-chains at the N and C-caps and the replacement of alanine by glycine or serine at solvent-exposed surfaces. J. Mol. Biol. 227:544–559. [DOI] [PubMed] [Google Scholar]
- 34.Minton, A. P. 2000. Implications of macromolecular crowding for protein assembly. Curr. Opin. Struct. Biol. 10:34–39. [DOI] [PubMed] [Google Scholar]