Abstract
Missense mutations in the membrane-binding actin-based motor protein, myosin-1a (Myo1a), have recently been linked to sensorineural deafness in humans. One of these mutations, E385D, impacts a residue in the switch II region of the motor domain that is present in virtually all members of the myosin superfamily. We sought to examine the impact of E385D on the function of Myo1a, both in terms of mechanochemical activity and ability to target to actin-rich microvilli in polarized epithelial cells. While E385D-Myo1a demonstrated actin-activated ATPase activity, the VMAX was reduced threefold relative to wild-type. Despite maintaining an active mechanochemical cycle, E385D-Myo1a was unable to move actin in the sliding filament assay. Intriguingly, when an enhanced-green-fluorescent-protein-tagged form of E385D-Myo1a was stably expressed in polarized epithelial cells, this mutation abolished the microvillar targeting normally demonstrated by wild-type Myo1a. Notably, these data are the first to suggest that mechanical activity is essential for proper localization of Myo1a in microvilli. These studies also provide a unique example of how even the most mild substitution of invariant switch II residues can effectively uncouple enzymatic and mechanical activity of the myosin motor domain.
Class I myosins are monomeric actin-based motors with the ability to interact directly with cellular membranes by virtue of highly basic regions in their C-terminal tail domains. Myo1a was one of the first vertebrate class I myosins discovered over 20 years ago when it was identified as a major component of the enterocyte brush border (BB) cytoskeleton (1). Although cell biological studies initially indicated that Myo1a expression was restricted to the intestinal tract (2), recent reports have demonstrated that Myo1a transcripts are present in mouse inner ear (3,4), and that mutations in Myo1a are linked to autosomal dominant nonsyndromic deafness (NSD) in humans (4). In a genetic screen of individuals with deafness that mapped to locus DFNA48, but not yet linked to a specific gene, eight families were found to harbor mutations in Myo1a (R93X, 116insS, V306M, E385D, G662E, G674D, S797F, S910P) (4). Although this large cluster of mutations suggests that Myo1a may be a leading contributor to NSD, the role of Myo1a in the inner ear and the impact of NSD mutations on Myo1a function remain uncharacterized.
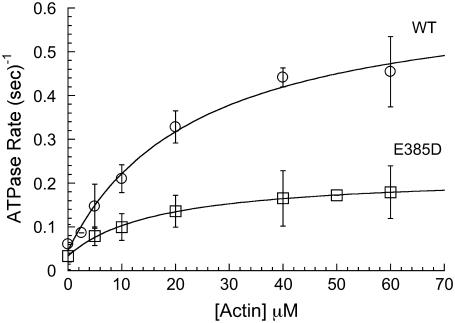
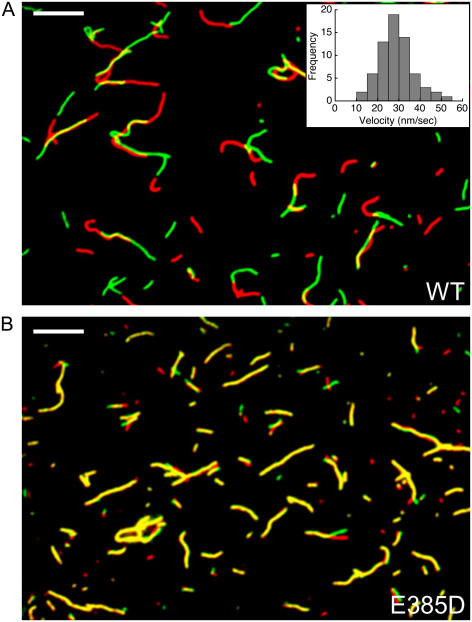
The goal of this study was to investigate the impact of the E385D mutation on the enzymatic and motor function of Myo1a. This substitution impacts an invariant glutamate in the active site (Fig. 1 A) and is associated with moderate-to-severe hearing loss with an early onset (4). To investigate the impact of E385D on Myo1a function, we produced wild-type (WT-Myo1a) and mutant (E385D-Myo1a) forms of full-length human Myo1a using a baculovirus/SF9 cell expression system. Both Myo1a variants were tagged with C-terminal tandem MYC-FLAG epitopes to facilitate purification and immobilization in motility assays. SDS-PAGE analysis showed that Myo1a migrated at the proper molecular weight, copurified with calmodulin, and was >95% pure based on Coomassie blue staining. As a first step in our functional analysis, we investigated the actin-activated ATPase activity of mutant and WT-Myo1a. E385D-Myo1a hydrolyzed ATP in an actin-dependent manner (VMax = 0.19 ± 0.01 s−1, KM = 17.0 ± 1.0 μM) albeit at a reduced rate relative to WT (VMax = 0.60 ± 0.05 s−1, KM = 24.0 ± 6.0 μM) (Fig. 2; data represent three preparations). The basal rates measured in this assay were similar (∼0.03–0.05 s−1). Actin cosedimentation assays demonstrated that E385D- and WT-Myo1a both have a low affinity for actin (KActin = 42 ± 5 and 73 ± 9 μM, respectively) in the presence of ATP (data not shown), suggesting E385D-Myo1a dissociates from actin in an ATP-dependent manner. Although these measurements demonstrate that E385D-Myo1a maintains a compromised yet active enzymatic cycle, we sought to determine the impact of this mutation on Myo1a mechanical activity. To this end, we measured the velocities produced by WT and E385D-Myo1a using a sliding filament assay. Before each assay, noncycling motors (“dead-heads”) were cleared from our preparations by ultracentrifugation in the presence of F-actin and ATP. In agreement with earlier studies (5), WT-Myo1a moved actin at 28.0 ± 1.0 nm/s at 23°C (mean ± SE, 66 filaments scored from three preparations; see inset, Fig. 3 A). Strikingly, the E385D mutation abolished actin movement. Coverslips coated with the mutant bound filaments at densities comparable to WT-Myo1a, yet filaments failed to undergo directed movement (Fig. 3 B). Although it remains possible that E385D-Myo1a moves actin at some exceedingly slow velocity, the speed would need to be at least 100-fold slower than WT to appear nonmotile on the timescale of our observations. Thus, these data indicate that E385D effectively uncouples the enzymatic and mechanical activities of Myo1a.
FIGURE 1.
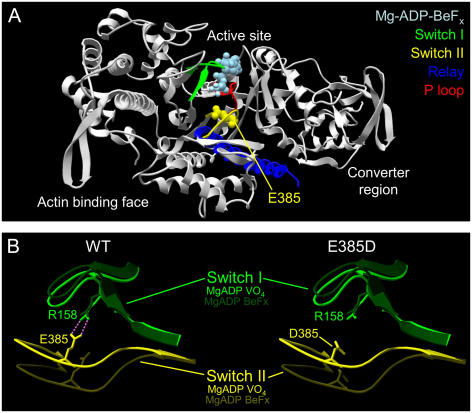
(A) Ribbon cartoon of the Dictyostelium myosin II motor domain showing the residue equivalent to Myo1a E385 relative to key structural elements of the active site. (B) Structural models of switch I and II in the ATP bound (Mg-ADP-BeFx) and transition state (Mg-ADP-VO4) show that the backdoor salt bridge (purple) may be disrupted in E385D. Modeling and bond detection (threshold of 3.3 Å) performed with DeepView v3.7. Myo1a residue numbers are shown.
FIGURE 2.
Actin-activated ATPase activity of WT and E385D Myo1a. Solid lines show the fit to a Michaelis-Menten model.
FIGURE 3.
(A) Overlay of fluorescent actin filaments imaged during a sliding filament assay with WT-Myo1a. Red represents filaments at t = 0, whereas green shows the state of each filament 310 s later. (B) E385D-Myo1a fails to produce actin sliding, resulting in near perfect overlap (yellow) in time shifted images. Bars are 10 μm in panels A and B.
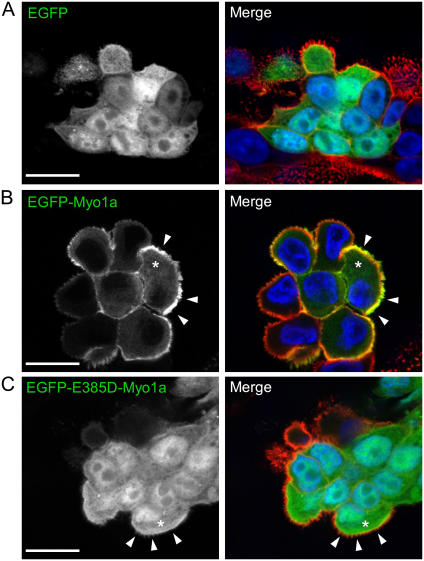
To understand how uncoupling mechanochemistry might influence the behavior of Myo1a in a cellular context, we expressed enhanced green fluorescent protein (EGFP)-tagged WT and E385-Myo1a variants in LLC-PK1-CL4 (CL4) cells. CL4 cells express a prominent apical brush border and form unique “cysts” that enable the observation of microvilli in a plane parallel to the image plane of the microscope (Fig. 4, A–C). Consistent with our previous studies (6), confocal microscopy revealed clear enrichment of EGFP-WT-Myo1a at the periphery of the cell in apical microvilli (arrows, Fig. 4 B). In contrast, EGFP-E385D-Myo1a demonstrated high cytosolic levels with fluorescence comparable to the cell periphery (compare asterisk with arrows, Fig. 4 C). The localization of EGFP-E385D-Myo1a was similar to EGFP alone (Fig. 4 A), indicating that this mutation disrupts the ability of Myo1a to localize to microvilli at the cell periphery.
FIGURE 4.
Expression of EGFP (A), EGFP-Myo1a (B), or EGFP-E385D-Myo1a (C) in CL4 cells. Images on the left show the EGFP signal alone; images on the right show the merge of EGFP (green) with phalloidin (red) and DAPI (blue) to visualize F-actin and nuclei, respectively. Bars are 20 μm in panels A–C. Images are representative of the localization observed in two independently derived stable lines for each construct.
E385 resides in a highly conserved region of the motor domain active site known as switch II (equivalent residue in Dictyostelium myosin-II is E459; Fig. 1, A and B) (7), where it may play a role in coordinating the water molecule that attacks the γ-phosphate during ATP hydrolysis (8). Indeed, structures of the myosin II motor domain crystallized with different nucleotide analogs reveal that the switch II region undergoes a large conformational change (>3 Å) between the ATP and transition state structures (8,9) (Fig. 1 B). During the transition state, E385 may also interact with R158 of switch I (equivalent residue in Dictyostelium myosin II is R238). This interaction may form a salt bridge that, along with the P-loop, constitutes the active site “backdoor” (Fig. 1 B), a structure believed to govern the rate of phosphate release following hydrolysis (10). Intriguingly, molecular modeling predicts that an aspartate at position 385 would be unable to form the backdoor salt bridge due to its shorter side chain (Fig. 1 B). Thus, whereas an E→D substitution would normally be considered a mild perturbation, the critical location of this residue may explain its ability to uncouple the enzymatic and mechanical activity of Myo1a. Indeed, previous studies on S456L, a switch II mutation in Dictyostelium myosin II, also described a mechanochemical uncoupling phenotype (11).
The mislocalization of E385D-Myo1a suggests that motor domain mechanical activity is required for targeting WT-Myo1a to microvilli. Intriguingly, these data appear to conflict with a previous study showing that the sole class I myosin from Aspergillus is able to fulfill essential cellular functions in the absence of enzymatic and mechanical activity (12). However, our new results are consistent with other work that highlights the significance of the motor domain in the subcellular targeting of class I myosins (13,14). Because our previous studies have also shown that the C-terminal tail is important for Myo1a localization (6), future studies must investigate how motor and tail domains cooperate to control subcellular localization.
These studies are the first to examine the impact of a human deafness mutation on the function of Myo1a. These results provide us with information on the molecular level defects that give rise to deafness in humans afflicted with the E385D mutation. Although the precise role of Myo1a within the inner ear is not clear, studies on the enterocyte brush border enable us to speculate that Myo1a is likely expressed in hair cells, which possess actin-rich stereocilia on their apex. Here, Myo1a may play a role in stabilizing apical membrane-cytoskeleton interactions, engage in intrastereociliar transport of membrane proteins or lipids, or power the shedding of apical membrane from stereocilia tips (15). Future studies must focus on elucidating the role of Myo1a in the context of the inner ear, as well as exploring the transient kinetic details that give rise to the mechanochemical uncoupling phenotype described here.
Acknowledgments
The authors thank members of the Tyska and Yengo laboratory groups for helpful suggestions. Images were acquired through the use of the Vanderbilt University Medical Center Cell Imaging Shared Resource.
This work was supported in part by grants from the March of Dimes (M.J.T.), the National Institutes of Health (DK075555 to M.J.T. and EY01750 to C.M.Y.), and American Heart Association (S.D.G., C.M.Y.).
Editor: Yale E. Goldman.
References
- 1.Mooseker, M. S., and R. E. Cheney. 1995. Unconventional myosins. Ann. Rev. Cell Dev. Biol. 11:633–675. [DOI] [PubMed] [Google Scholar]
- 2.Skowron, J. F., W. M. Bement, and M. S. Mooseker. 1998. Human brush border myosin-I and myosin-Ic expression in human intestine and Caco-2BBe cells. Cell Motil. Cytoskeleton. 41:308–324. [DOI] [PubMed] [Google Scholar]
- 3.Dumont, R. A., Y. D. Zhao, J. R. Holt, M. Bahler, and P. G. Gillespie. 2002. Myosin-I isozymes in neonatal rodent auditory and vestibular epithelia. J. Assoc. Res. Otolaryngol. 3:375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donaudy, F., A. Ferrara, L. Esposito, R. Hertzano, O. Ben-David, R. E. Bell, S. Melchionda, L. Zelante, K. B. Avraham, and P. Gasparini. 2003. Multiple mutations of MYO1A, a cochlear-expressed gene, in sensorineural hearing loss. Am. J. Hum. Genet. 72:1571–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins, K., J. R. Sellers, and P. Matsudaira. 1990. Calmodulin dissociation regulates brush border myosin I (110-kD- calmodulin) mechanochemical activity in vitro. J. Cell Biol. 110:1137–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyska, M. J., and M. S. Mooseker. 2002. MYO1A (brush border myosin I) dynamics in the brush border of LLC-PK1–CL4 cells. Biophys. J. 82:1869–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cope, M. J. T., J. Whisstock, I. Rayment, and J. Kendrick-Jones. 1996. Conservation within the myosin motor domain: implications for structure and function. Structure. 4:969–987. [DOI] [PubMed] [Google Scholar]
- 8.Smith, C. A., and I. Rayment. 1996. X-ray structure of the magnesium(II).ADP.vanadate complex of the Dictyostelium discoideum myosin motor domain to 1.9 A resolution. Biochemistry. 35:5404–5417. [DOI] [PubMed] [Google Scholar]
- 9.Fisher, A. J., C. A. Smith, J. B. Thoden, R. Smith, K. Sutoh, H. M. Holden, and I. Rayment. 1995. X-ray structures of the myosin motor domain of Dictyostelium discoideum complexed with MgADP.BeFx and MgADP.AlF4-. Biochemistry. 34:8960–8972. [DOI] [PubMed] [Google Scholar]
- 10.Lawson, J. D., E. Pate, I. Rayment, and R. G. Yount. 2004. Molecular dynamics analysis of structural factors influencing back door pi release in myosin. Biophys. J. 86:3794–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy, C. T., R. S. Rock, and J. A. Spudich. 2001. A myosin II mutation uncouples ATPase activity from motility and shortens step size. Nat. Cell Biol. 3:311–315. [DOI] [PubMed] [Google Scholar]
- 12.Liu, X., N. Osherov, R. Yamashita, H. Brzeska, E. D. Korn, and G. S. May. 2001. Myosin I mutants with only 1% of wild-type actin-activated MgATPase activity retain essential in vivo function(s). Proc. Natl. Acad. Sci. USA. 98:9122–9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang, N., and E. M. Ostap. 2001. Motor domain-dependent localization of myo1b (myr-1). Curr. Biol. 11:1131–1135. [DOI] [PubMed] [Google Scholar]
- 14.Ruppert, C., J. Godel, R. T. Muller, R. Kroschewski, J. Reinhard, and M. Bahler. 1995. Localization of the rat myosin I molecules myr 1 and myr 2 and in vivo targeting of their tail domains. J. Cell Sci. 108:3775–3786. [DOI] [PubMed] [Google Scholar]
- 15.McConnell, R. E., and M. J. Tyska. 2007. Myosin-1a powers the sliding of apical membrane along microvillar actin bundles. J. Cell Biol. 177:671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]