Abstract
The influence of the local anesthetic lidocaine on electrostatic properties of a lipid membrane bilayer was studied by molecular dynamics simulations. The electrostatic dipole potential, charge densities, and orientations of the headgroup angle have been examined in the presence of different amounts of charged or uncharged forms of lidocaine. Important changes in the membrane properties caused by the presence of both forms of lidocaine are presented and discussed. Our simulations have shown that both charged and uncharged lidocaine cause almost the same increase in the electrostatic potential in the middle of the membrane, although for different reasons. The increase, ∼90 mV for 9 mol % of lidocaine and 220 mV for 28 mol % of lidocaine, is of a size that may affect the functioning of voltage-gated ion channels.
INTRODUCTION
Local anesthetics are a well-known group of pharmaceutical agents used to relieve pain in specific parts of the organism, inhibiting propagation of signals along the nerves. Despite the fact that local anesthetics have a very important field of application and have been used in medical treatment for more than 50 years, the molecular mechanism of their action remains almost unknown (1). A logical extension of the observations by Meyer and Overton (2,3) that the therapeutic potency of anesthetics is correlated with the partition coefficient in olive oil is that local anesthetics act by targeting the cell membrane. It is known experimentally that anesthetic molecules are able to block Na+ ion channels in neuronal cells (4–7); thus, one can suggest that the anesthetics cause changes in the membrane or in the membrane proteins that affect functioning of ion channels. In recent decades, protein-oriented theories claiming that binding of anesthetic molecules to specific binding sites in membrane protein is responsible for the anesthetic effect have prevailed (8–11), and a binding site for some local anesthetics in the voltage-gated sodium channel has been proposed (12). On the other hand, the strong focus of protein-oriented theories on an anesthetic mechanism in terms of local anesthetic interaction with a binding site, not taking the lipid surrounding into account, has been criticized (13,14).
Several different mechanisms through which the presence of local anesthetics in lipid bilayers can modulate conductivity of ion channels have been suggested. One plausible mechanism is that the electrostatic dipole potential that arises from the oriented dipoles at the membrane-water interface, which changes on addition of anesthetics, could regulate voltage-gated ion channels (13). Among other mechanisms under discussion are changes in the bilayer lateral pressure profile, which could shift the equilibrium between the active and inactive forms of membrane proteins (13,15), increased membrane fluidity (manifested also in the decrease of the phase transition temperature (14)), changes in lipid hydration (14), and specific hydrogen bond formation (16).
Lidocaine is one of the most common amide-type local anesthetics. In aqueous solution lidocaine usually exists as a mixture of charged and uncharged species, with a pKa value estimated as 7.9 (17,18). It is believed that the charged form is responsible for the therapeutic action (8). It has been found that the membrane-water partition coefficient of the uncharged form is higher than that of the charged form by 1.15 (17), which indicates that inside membranes, the balance between the charged and uncharged forms is shifted in favor of the uncharged species. The role of the neutral form of lidocaine may thus also be important because of its greater ability to penetrate inside membranes (18).
In this article, we use molecular dynamics simulations to investigate the effects of the charged and uncharged forms of lidocaine on the electrostatic properties of a model lipid membrane. Molecular dynamics simulations enable us to get a very detailed picture of the molecular events and thus provide us with a unique tool to understand phenomena at the molecular level. During the last decade, molecular dynamics has been extensively used to study many properties of lipid bilayers (19–24) including the cases in which bilayer-associated molecules were present (25–27).
In our previous work (28), we studied preferential location and orientation of the charged and uncharged lidocaine in dimyristoylphosphatidylcholine (DMPC) bilayers as well as its hydration properties. The analysis of the work we present here is concentrated on the changes in the lipid bilayer that are caused by the both forms of lidocaine and that are of importance for the membrane electrostatic properties. In addition to the simulations performed in the previous work (28), simulations at three times higher lidocaine concentration have been carried out and analyzed to highlight the effect on membrane caused by the presence of lidocaine.
COMPUTATIONAL METHODS
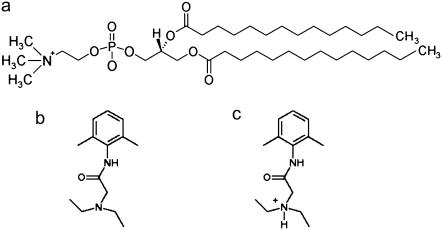
Five different lipid bilayer systems, each consisting of 128 DMPC lipids and 3655 water molecules, were simulated. In addition to the molecules mentioned above, two of the systems also contained either 12 charged or 12 uncharged lidocaine molecules, and two other systems contained either 36 charged or 36 uncharged lidocaine molecules. Molecular structures of DMPC and the two forms of lidocaine are shown in Fig. 1. To keep electroneutrality, 12 or 36 Cl− ions were added to the corresponding systems with charged lidocaine. One system containing a pure fully hydrated DMPC bilayer was simulated as a reference. The molecules were described within the united atom GROMOS force field (except the polar H atom on charged lidocaine, which was described explicitly), with the interaction parameters included in the GROMACS simulation package (29). The temperature was set to 313 K and the pressure to 1 bar. The long-range electrostatic forces were treated using the particle mesh Ewald technique (30). Further details on the simulation setup, partial atom charges on lidocaine, system preparation, etc., can be found in our previous article (28).
FIGURE 1.
Molecular structures used in the simulations. (a) DMPC, (b) uncharged lidocaine, and (c) charged lidocaine.
All simulations containing lidocaine were run 100 ns from the starting conditions. The last 50 ns were used for trajectory analysis. The reference system, with a start configuration taken from a previous well-equilibrated simulation (24), was simulated 50 ns from which the last 45 ns were used for trajectory analysis. All simulations were carried out using the GROMACS v.3.2 simulation package (29).
For calculation of the electrostatic potential we start from the Poisson equation:
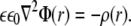 |
(1) |
Here, 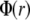 is the electrostatic potential, and
is the electrostatic potential, and 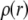 is the charge density. The dielectric constant ɛ in atomistic simulations is set to 1.
is the charge density. The dielectric constant ɛ in atomistic simulations is set to 1.
Because of translational symmetry in the X and Y directions, the electrostatic potential and the charge density depend only on the Z coordinate. From a simulation we can construct the charge distribution by slicing the Z direction of the membrane into thin slices and sum for all partial atom charges in each slice. By using the boundary conditions 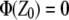 and
and 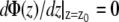 in reference point z0, here chosen to be in the middle of the water layer, we can get the electrostatic potential by integrating the Poisson equation over the box z coordinate:
in reference point z0, here chosen to be in the middle of the water layer, we can get the electrostatic potential by integrating the Poisson equation over the box z coordinate:
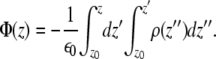 |
(2) |
RESULTS AND DISCUSSION
Area per lipid
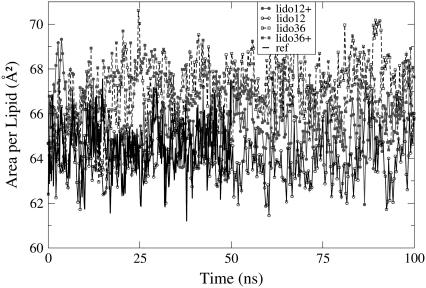
The average area per lipid is a fundamental property of lamellar bilayer systems. Many other properties depend on it to some extent. Here we use the area per lipid to monitor equilibration of the simulated systems. The time evolution of the area per lipid for each of the simulated systems is shown in Fig. 2. No visible trends are seen. Block averaging over the last 50 ns of the trajectory shows no drift in the evolution of the area per lipid for the simulated systems, and consequently, the systems were regarded to be in equilibrium.
FIGURE 2.
Evolution of the area per lipid. Charged lidocaine, lines with solid symbols; uncharged, lines with open symbols; 12 lidocaines, solid lines with circles; 36 lidocaines, dashed lines with squares; reference system, black line without symbols.
In Table 1 we present mean values of the calculated areas per lipid. Simulations in the presence of lidocaine show larger areas per lipid, and the previously noticed trend that the area per lipid in the system with charged lidocaine is slightly smaller than in the corresponding system with uncharged lidocaine (28) holds even for the larger lidocaine concentration.
TABLE 1.
Some properties of the simulated systems
| Simulated system | Area per lipid (Å2) | Φ(0) (V) | Φmax (V) | Headgroup angle (°) |
|---|---|---|---|---|
| Reference | 64.2 | 0.560 | 0.76 | 79.8 |
| 12 lidocaine | 65.4 | 0.656 | 0.74 | 79.0 |
| 12 charged lidocaine | 64.4 | 0.651 | 0.80 | 72.2 |
| 36 lidocaine | 67.6 | 0.770 | 0.73 | 79.0 |
| 36 charged lidocaine | 66.8 | 0.781 | 0.84 | 60. |
| Uncertainty | 0.15 | 0.002 | 0.01 | 0.5 |
Φ(0) is the electrostatic potential in the middle of membrane, and Φmax is its maximal value. For other details, see the text.
Electrostatic potential
The electrostatic potential across a membrane is an important property of lipid bilayers that may be relevant for understanding the mechanisms behind the functioning of ion channels. The potential arises as a result of specific preferential orientations of the lipid headgroup dipoles and water dipoles at the membrane-water interface. For this reason, it is often referred to as the bilayer dipole potential. The presence of ions and other charged species affects the electrostatic potential too.
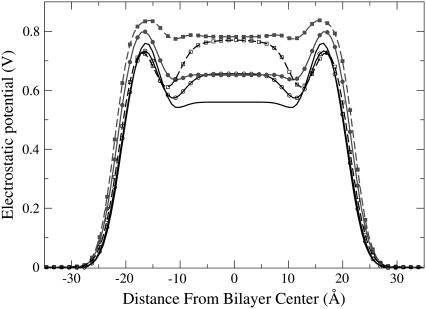
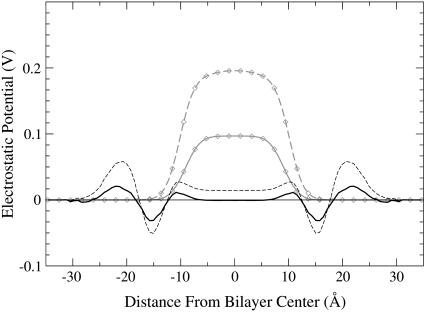
In Fig. 3, the total electrostatic potential is presented for all five simulations. In Table 1, the values of the potential in the middle of the membrane and the maximum values of the potential are given. The overall picture is in agreement with previous simulations using the GROMACS force field, showing positive dipole potential 500–600 mV in the middle of the bilayer (26,31). Accurate experimental determination of the dipole potential is difficult, and different sources report different values. Many reported experiments provide values of the potential in the middle of the membrane in the range 300–800 mV (32). Other experimental studies have suggested a lower value for the dipole potential in the range 220–280 mV (33), and a value of 510 mV has recently been reported for diphytanoylphosphatidylcholine (ester-DPhPC) membrane (34), which is close to our result for the reference system.
FIGURE 3.
Electrostatic potential. Charged lidocaine, lines with solid symbols; uncharged, lines with open symbols; 12 lidocaines, solid lines with circles; 36 lidocaines, dashed lines with squares; reference system, black line without symbols.
It can be seen that both charged and uncharged lidocaine have a pronounced influence on the potential. In the headgroup region, one can see a slight decrease of the electrostatic potential by ∼30 mV for the uncharged lidocaine at both concentrations. For the charged systems, the potential in the headgroup region is increased by 46 mV and 76 mV for the simulations with 12 and 36 lidocaine molecules, respectively. More interesting is the change of the potential for the hydrocarbon region of the lipid tails. The striking result is that the potentials in the middle part of the bilayer are almost the same for charged and uncharged forms of lidocaine at the two concentrations. Relative to the reference bilayer, the potential is increased by ∼93 mV for simulations with 12 lidocaine molecules and by 220 mV for simulations with 36 lidocaine molecules.
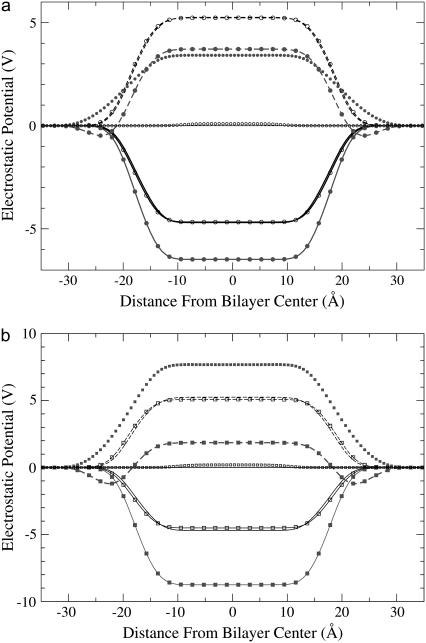
The fact that positively charged lidocaine increases the electrostatic potential inside a membrane is quite natural. It is remarkable that the uncharged lidocaine does the same. To get more insight into this effect, we displayed contributions to the electrostatic potential from DMPC, water, lidocaine, and Cl− ions (see Fig. 4). For the reference membrane, the resulting potential is obtained as a sum of a positive potential from water and a negative one from lipids, each contribution exceeding the resulting potential by about one order of magnitude. In the presence of charged lidocaine, a strong positive contribution appears from the positive lidocaine and negative Cl− ions, which in the middle of the membrane reaches values of 3.42 V and 7.68 V for the systems with 12 and 36 lidocaine+, respectively (solid symbols in Fig. 4). Simultaneously, one can observe a clear decrease of the potential coming from both DMPC and water (solid and dashed lines with solid symbols in comparison with the corresponding lines without symbols). For DMPC, there are decreases by 1.85 V and 4.06 V, and for water by 1.45 V and 3.36 V, for 12 and 36 lidocaine+, respectively. For water, a small dip in the potential can be also seen at the bilayer interface. The dip is more pronounced for the system with 36 lidocaine molecules. It reflects the change in preferential orientation of water dipoles in the outer surface area of the membrane as a result of their interactions with Cl− ions. Most of the observed changes of the potential coming from different components cancel each other, resulting in a rather moderate increase of the total electrostatic potential.
FIGURE 4.
Different contributions to the electrostatic potential. (a) 12 lidocaine molecules; (b) 36 lidocaine molecules. DMPC, solid lines; water, dashed lines; lidocaine (with Cl− ions if charged), symbols without line; uncharged systems, open symbols; charged systems, solid symbols; reference system, lines without symbols.
In the case of uncharged lidocaine, the picture is different. Neither water nor DMPC contributions to the electrostatic potential change noticeably (compare lines without symbols and lines with open symbols in Fig. 4). To illustrate this more clearly, in Fig. 5 we display the contribution to the dipole potential coming from the uncharged lidocaine only (shaded lines with diamonds), in comparison with the contribution of water and lipids to the total change of the potential (lines without symbols). It is clear that almost the whole contribution to the total change of the dipole potential comes from the lidocaine alone. The most strongly charged atom on a neutral lidocaine molecule is the negatively charged carbonyl oxygen (the charge is −0.41), and the rest of the molecule has mainly a weak positive charge. In our previous work (28), we found that the preferential location of the uncharged lidocaine is just below the headgroups with orientation parallel to the bilayer surface. In this coordination, the carbonyl oxygen can orient itself interacting favorably with polar atoms of the headgroups, whereas the “back” side of lidocaine interacts with the upper parts of the apolar lipid tails. Our analysis shows that the most probable value of the angle between the carbonyl CO vector of the uncharged lidocaine and the bilayer normal is between 30° and 40°. Such preferential orientation of the uncharged lidocaine may create a small but noticeable contribution to the total electrostatic potential.
FIGURE 5.
Contribution of the uncharged lidocaine to the total electrostatic potential (shaded lines with diamonds) and contribution of water and lipids to the change of the electrostatic potential on addition of lidocaine (lines without symbols); 12 lidocaines, solid lines; 36 lidocaines, dashed lines.
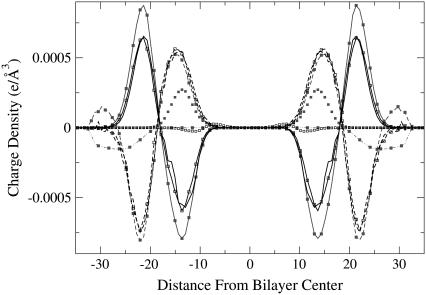
Charge density
Because the electrostatic potential is determined by the distribution of different charged groups, to get better insight into the origin of the observed changes, we have plotted the charge density for different components in Fig. 6 as a function of the box z coordinate. For the reference system, the charges from the choline and phosphate groups are compensated by the presence of water. The water contribution to the charge density (dashed lines) is slightly greater than that of the lipids (solid lines), which leads to a total positive potential in the middle of the membrane. One can see that the density distribution from the positively charged lidocaine (solid squares without connection lines) is not overlapping with the negative distribution from Cl− ions (solid squares with dot-dashed line). This “double layer” creates a large positive potential if one considers the contribution from lidocaine+ and Cl− only (see Fig. 4). However, the positive charge density of the charged lidocaine is largely compensated by the change of the negative charge density from the lipid phosphate groups, whereas the negative contribution of Cl− ions is mostly compensated by water and choline groups of the lipids. Such compensation of charges can be interpreted as dielectric screening of charged species (lidocaine+ and Cl−) by dipoles of water and lipid headgroups. From the numerical data cited in the previous section, the increase of the total electrostatic potential is reduced by a factor of 37 for 12 lidocaine molecules and by a factor of 35 for 36 lidocaine molecules, compared with the increase coming from the charged components only (charged lidocaine+ and Cl− ions). The cited factor can be viewed as an effective dielectric permittivity of the water-lipid interface, and one can see that the dielectric response is nearly linear in the considered concentration range.
FIGURE 6.
Contributions to the charge density from different components in the systems with 36 lidocaine molecules and in the reference system. DMPC, solid lines; water, dashed lines; lidocaine, dots; Cl− ions, dot-dashed line; uncharged systems, open symbols; charged systems, solid symbols; reference system, lines without symbols.
In the case of uncharged lidocaine molecules, the water and lipid charge densities remain mostly unperturbed, and the major part of the change of the total electrostatic potential comes from a fluctuation of the lidocaine charge density only, which is seen in Fig. 6 as open squares in the range 6–14 Å from the bilayer center. It is just this density that creates a positive change of the total dipole potential, and water and lipids do not contribute to the change of the dipole potential in the middle of membrane, as seen in Fig. 5.
The observed changes of the electrostatic potential are in the same magnitude range as the transmembrane potential for a cell membrane in vivo (32) in the system with 12 lidocaine molecules (∼9 mol % concentration). This indicates that such changes could be a plausible mechanism for the action of local anesthetics. By changing the distribution of the potential inside the membrane, the neuron may be blocked from reaching its threshold value and thus prevented from working properly. Note also that a positive change of the electrostatic potential in the middle of membrane of the order of a few kT units (which can also propagate inside an ion channel because of the long-range character of electrostatic interactions) creates an additional energy barrier for cations to pass through the membrane.
Headgroup angle
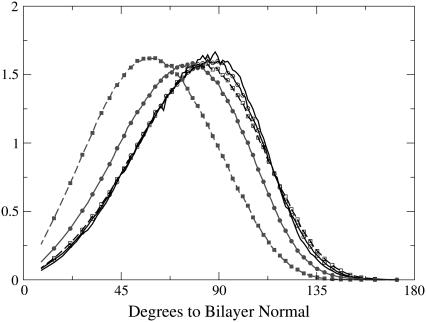
It is clear from the data presented above that the most significant changes in the bilayer caused by addition of lidocaine occur in the headgroup region. Also, behavior of the headgroup dipoles is the main factor behind the membrane electrostatics. We therefore analyzed how the presence of the lidocaine affects the angular distribution of the phosphorus-nitrogen (P-N) vector relative to the bilayer normal, which is assumed to be parallel to the Z axis of the simulation box. Fig. 7 shows the distribution of the P-N vector for each monolayer separately relative to the normal vector directed out of the bilayer. Average values of the P-N tilt angle (the angle between P-N vector and the bilayer normal) are also given in Table 1.
FIGURE 7.
Distribution of the angle between the phosphorus-to-nitrogen vector and the bilayer normal for each of the leaflets; the normal is directed out of the bilayer. Charged lidocaine, lines with solid symbols; uncharged, lines with open symbols; 12 lidocaines, lines with circles; 36 lidocaines, lines with squares; reference system, line without symbols.
From Fig. 7, it can be seen that the uncharged lidocaine has almost no influence on the orientation of the headgroup. The computed average headgroup tilt angle has a value of 79.8° for the reference bilayer, to be compared with 79.0° for the system with 12 and 36 uncharged lidocaines, the difference being of the order of the statistical uncertainty. For the systems with charged lidocaine the effect is more dramatic: the average tilt angle decreases to 72° for 12 lidocaine+ and down further to 60° for 36 lidocaine+. Similar trends are seen in the angular distribution: whereas the uncharged lidocaine leaves the distributions almost intact, centered at the angle almost parallel to the membrane surface with a slight preference for the direction out of the membrane, the charged lidocaine causes noticeable reorientation of the headgroup vectors toward the water phase. Previously, a change of the P-N angle has been suggested as an example of a “molecular voltmeter” (35) defining the membrane dipole potential. If we come back to the dipole potential profiles (Fig. 3), we see that in the headgroup region, up to the ester groups, the dipole potential for systems with the uncharged lidocaine mostly coincides with that of the reference system, whereas for the charged lidocaine, we see an increase of the dipole potential in the headgroup region. This effect clearly correlates with the observed changes in the P-N angle and is in fact not very surprising considering the ionic distribution in water near the bilayer surface, which attracts the positively charged choline groups. Under these circumstances, it is energetically advantageous for the headgroup to change its orientation. Moreover, the effect from repulsion between the positive charge on lidocaine molecules, located at the level of phosphate and ester groups, and the positive charge on the choline group makes the decrease of the angle favorable. The uncharged lidocaine is coordinated mostly under the ester groups and in the upper parts of the lipid tails and does not affect charge distribution in the headgroup region, causing an increase of the electrostatic potential only in the tail region of the membrane.
The observed behavior of the headgroup tilt angle is in agreement with the presented results for the charge distribution and the electrostatic potential.
CONCLUSIONS
In this article we have examined the influence of charged and uncharged lidocaine on electrostatic properties of a lipid bilayer, which may be of importance for an understanding of lidocaine anesthetic action. We have analyzed the electrostatic potential, charge distributions, and the headgroup tilt angle. All examined properties showed significant changes of different character in the presence of either charged or uncharged lidocaine molecules.
The dipole electrostatic potential was found to be affected by the presence of both forms of lidocaine. A very interesting observation is that the electrostatic potential in the lipid tail region turned out to be almost the same for both charged and uncharged lidocaine at equal concentrations. The mechanism of the change of the electrostatic potential is, however, very different for the two forms. The charged lidocaine, together with neutralizing Cl− ions, has a strong influence on the behavior of the lipid headgroups, leading to a decrease of the tilt of the phosphorus-nitrogen dipole vector and generally causing a serious rearrangement of the charges of all molecular species involved. Most of changes in the charge distributions cancel each other, resulting in a moderate increase of the total electrostatic potential inside the membrane. The uncharged lidocaine keeps the lipid structure and associated charge distribution almost intact. The total electrostatic potential in the middle of the membrane increases in this case because of partial charges on the lidocaine itself, with the main contribution from dipole moment of the carbonyl group.
Also, for the headgroup angle we see significant changes in the presence of charged lidocaine that could influence membrane protein functioning. We note that similar effects, including change of the headgroup tilt angle and increase of the dipole potential, have been observed for cationic lipids (36) that are not known to have anesthetic effects. Thus, there can be other molecules that do not cause anesthesia but do cause similar molecular effects. On the other hand, there is an observation that addition of positively charged lipids and their analogs suppress activity of K+ channels (37).
The changes of the membrane dipole potential observed in the work presented here are of the order (and even higher) of typical values of the transmembrane potentials and may probably affect functioning of the voltage-gated ion channels. Moreover, the direction of the change, an increase, creates an additional barrier for cations to penetrate through the membrane. It therefore seems plausible that the presence of lidocaine causes blocking of Na+ ion channels through a change of the electrostatic potential. Because of the difference in their partition coefficients, the uncharged form of lidocaine should be predominant (at neutral pH) in the membrane interior compared to the charged form. It is important in this connection that even uncharged lidocaine causes an increase of the potential in the middle of the membrane. It is also worth noting that the observed increase of the electrostatic potential in the presence of uncharged lidocaine takes place mostly as a result of partial charges associated with the carbonyl group in the middle of the molecule. Such a carbonyl group is also present in many other local anesthetics such as tetracaine, procaine, or bupivacaine.
Acknowledgments
This work has been supported by the Swedish Research Council (Vetenskapsrådet). The computing facilities have been granted by the Center for Parallel Computing at the Royal Institute of Technology, Stockholm, and the High-Performance Computer Center at Umeå University.
Editor: Gregory A. Voth.
References
- 1.Eckenhoff, R. G. 2001. Promicuous ligands and attractive cavities: how do the inhaled anesthetics work? Mol. Interv. 1:258–268. [PubMed] [Google Scholar]
- 2.Meyer, K. H. 1937. Contribution to the theory of narcosis. Trans. Faraday Soc. 33:1060–1068. [Google Scholar]
- 3.Overton, C. E. 1901. Studien über die Narkose, Zugleich ein Beitrag zur Allegemeiner Pharmakologie. Gustav Fischer, Jena, Switzerland. Translation: 1990. Studies of Narcosis. Chapman and Hall, London.
- 4.Hille, B. 1977. Local anesthetics: Hydrophilic and hydrophobic pathways for the drug-receptor reaction. J. Gen. Physiol. 69:497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilius, B., K. Benndorf, and F. Markwardt. 1987. Effects of lidocaine on single cardiac sodium-channels. J. Mol. Cell. Cardiol. 19:865–874. [DOI] [PubMed] [Google Scholar]
- 6.Butterworth, J. F., and G. R. Strichartz. 1990. Molecular mechanisms of local anesthesia: a review. Anesthesiology. 72:711–734. [DOI] [PubMed] [Google Scholar]
- 7.Weiser, T. 2006. Comparison of the effect of four Na+ channel analgesics on TTX-resistant Na+ currents in rat sensory neurons and recombinant Mav 1.2 channels. Neurosci. Lett. 395:179–184. [DOI] [PubMed] [Google Scholar]
- 8.Narahashi, T., D. T. Frazier, and M. Yamada. 1969. Cationic forms of local anesthetics block action potentials from inside nerve membrane. Nature. 223:748–749. [DOI] [PubMed] [Google Scholar]
- 9.Franks, N. P., and W. R. Lieb. 1994. Molecular and cellular mechanisms of general anestesia. Nature. 367:607–614. [DOI] [PubMed] [Google Scholar]
- 10.Sheets, M. F., and D. A. Hanck. 2003. Molecular action of lidocaine on the voltage sensors of sodium channels. J. Gen. Physiol. 121:163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipkind, G. M., and H. A. Fozzard. 2005. Molecular modeling of local anesthetic drug binding by voltage-gated sodium channels. Mol. Pharmacol. 68:1611–1622. [DOI] [PubMed] [Google Scholar]
- 12.Ragdale, D. S., J. C. McPhee, T. Scheuer, and W. A. Catterall. 1994. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 265:1724–1728. [DOI] [PubMed] [Google Scholar]
- 13.Cafiso, D. S. 1998. Dipole potentials and spontaneous curvature: membrane properties that could mediate anesthesia. Toxicol. Lett. 100–101:431–439. [DOI] [PubMed] [Google Scholar]
- 14.Ueda, I., and T. Yoshida. 1999. Hydration of lipid membranes and the action mechanisms of anesthetics and alcohols. Chem. Phys. Lipids. 101:65–79. [DOI] [PubMed] [Google Scholar]
- 15.Cantor, R. S. 1999. The influence of membrane lateral pressures on simple geometric models of protein conformational equlibria. Chem. Phys. Lipids. 101:45–56. [DOI] [PubMed] [Google Scholar]
- 16.da Motta Neto, J. D., and R. B. de Alencastro. 1997. Theoretical studies on local anesthetics: procaine, lidocaine, tetracaine, bupivacaine, and dibucaine—neutral and monoprotonated. Int. J. Quant. Chem. 61:959–980. [Google Scholar]
- 17.Avdeef, A., K. J. Box, J. E. A. Comer, C. Hibbert, and K. Y. Tam. 1998. pH-metric logP10. Determination of liposomal membrane-water partition coefficients of ionizable drugs. Pharm. Res. 15:209–215. [DOI] [PubMed] [Google Scholar]
- 18.Matsuki, H., M. Yamanaka, H. Kamaya, S. Kaneshina, and I. Ueda. 2005. Dissociation equilibrium between uncharged and charged local anesthetic lidocaine in a surface-adsorbed film. Colloid Polym. Sci. 283:512–520. [Google Scholar]
- 19.Pastor, R. W. 1994. Computer simulations of lipid bilayers. Curr. Opin. Struct. Biol. 4:486–492. [Google Scholar]
- 20.Tieleman, D. P., and H. J. C. Berendsen. 1996. Molecular dynamics simulations of a fully hydrated dipalmitoylphosphatidylcholine bilayer with different macroscopic boundary conditions and parameters. J. Chem. Phys. 105:4871–4880. [Google Scholar]
- 21.Berger, O., O. Edholm, and F. Jahnig. 1997. Molecular dynamics simulations of a fluid bilayer of dipalmitoylphosphatidylcholine at full hydration, constant pressure and constant temperature. Biophys. J. 72:2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott, H. L. 2002. Modeling the lipid component of membranes. Curr. Opin. Struct. Biol. 12:495–502. [DOI] [PubMed] [Google Scholar]
- 23.Lopes, F. L., S. O. Nielsen, and M. L. Klein. 2004. Hydrogen bonding structure and dynamics of water at the dipalmitoylphosphatidylcholine lipid bilayer surface from a molecular dynamics simulation. J. Phys. Chem. B. 108:6603–6610. [Google Scholar]
- 24.Högberg, C. J., and A. P. Lyubartsev. 2006. A molecular dynamics investigation of the influence of hydration and temperature on structural and dynamical properties of a dimyristoylphosphatidylcholine bilayer. J. Phys. Chem. B. 110:14326–14336. [DOI] [PubMed] [Google Scholar]
- 25.Falck, E., M. Patra, M. Karttunen, M. T. Hyvönen, and I. Vattulainen. 2004. Lessons of slicing membranes: Interlplay of packing, free area, and lateral duffusion in phospholipid/cholesterol bilayers. Biophys. J. 87:1076–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patra, M., E. Salonen, E. Terama, I. Vattulainen, R. Faller, B. W. Lee, J. Holopainen, and M. Karttunen. 2006. Under the influence of alcohol: The effect of ethanol and methanol on lipid bilayers. Biophys. J. 90:1121–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedersen, U. R., G. H. Peters, and P. Westh. 2007. Molecular packing in 1-hexanol-DMPC bilayers studied by molecular dynamics simulations. Biophys. Chem. 125:104–111. [DOI] [PubMed] [Google Scholar]
- 28.Högberg, C. J., A. Maliniak, and A. P. Lyubartsev. 2007. Dynamical and structural properties of charged and uncharged lidocaine in a lipid bilayer. Biophys. Chem. 125:416–424. [DOI] [PubMed] [Google Scholar]
- 29.Lindahl, E., B. Hess, and D. van der Spoel. 2001. GROMACS 3.0: A package for molecular simulations and trajectory analysis. J. Mol. Model. 7:306–317. [Google Scholar]
- 30.Darden, T. A., D. York, and L. Pedersen. 1993. Particle mesh Ewald: An NlogN method for Ewald sums in large systems. J. Chem. Phys. 98:10089–10092. [Google Scholar]
- 31.Wohlert, J., and O. Edholm. 2004. The range and shielding of dipole-dipole interactions in phospholipid bilayers. Biophys. J. 87:2433–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cevc, G. 1990. Membrane electrostatics. Biochim. Biophys. Acta. 1031:311–382. [DOI] [PubMed] [Google Scholar]
- 33.Clarke, R. J. 2001. The dipole potential of phospholipid membranes and methods for its detection. Adv. Colloid Interface Sci. 89–90:263–281. [DOI] [PubMed] [Google Scholar]
- 34.Wang, L., P. S. Bose, and F. J. Sigworth. 2006. Using cryo-EM to measure the dipole potential of a lipid membrane. Proc. Natl. Acad. Sci. USA. 103:18528–18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seelig, J., P. M. Macdonald, and P. G. Scherer. 1987. Phospholipid head group as sensors of electric charge in membranes. Biochemistry. 26:7535–7541. [DOI] [PubMed] [Google Scholar]
- 36.Gurtovenko, A. A., M. Patra, M. Karttunen, and I. Vattulainen. 2004. Cationic DMPC/DMPTAP lipid bilayers: molecular dynamics study. Biophys. J. 86:3461–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pertou, S., R. W. Ordway, J. A. Hamilton, J. V. Walsh, Jr., and J. J. Singer. 1994. Structural requirements for charged lipid molecules to directly increase or suppress K+ channel activity in smooth muscle cells. J. Gen. Physiol. 103:471–486. [DOI] [PMC free article] [PubMed] [Google Scholar]