Abstract
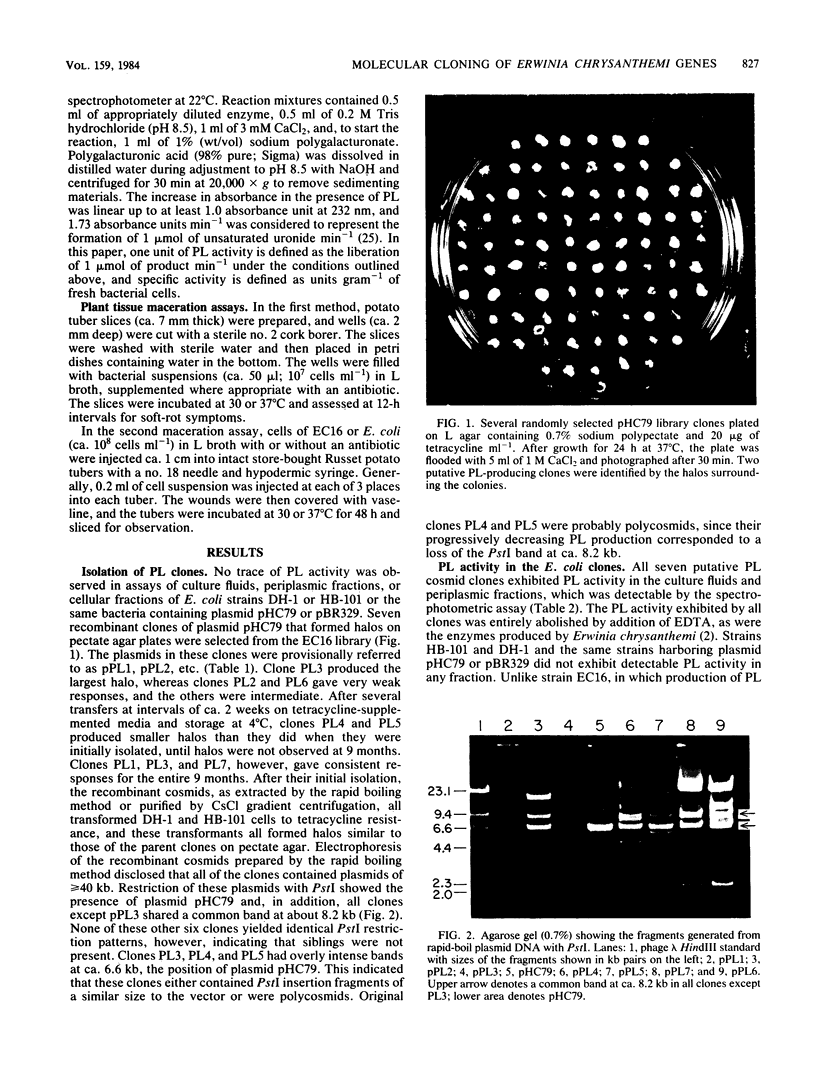
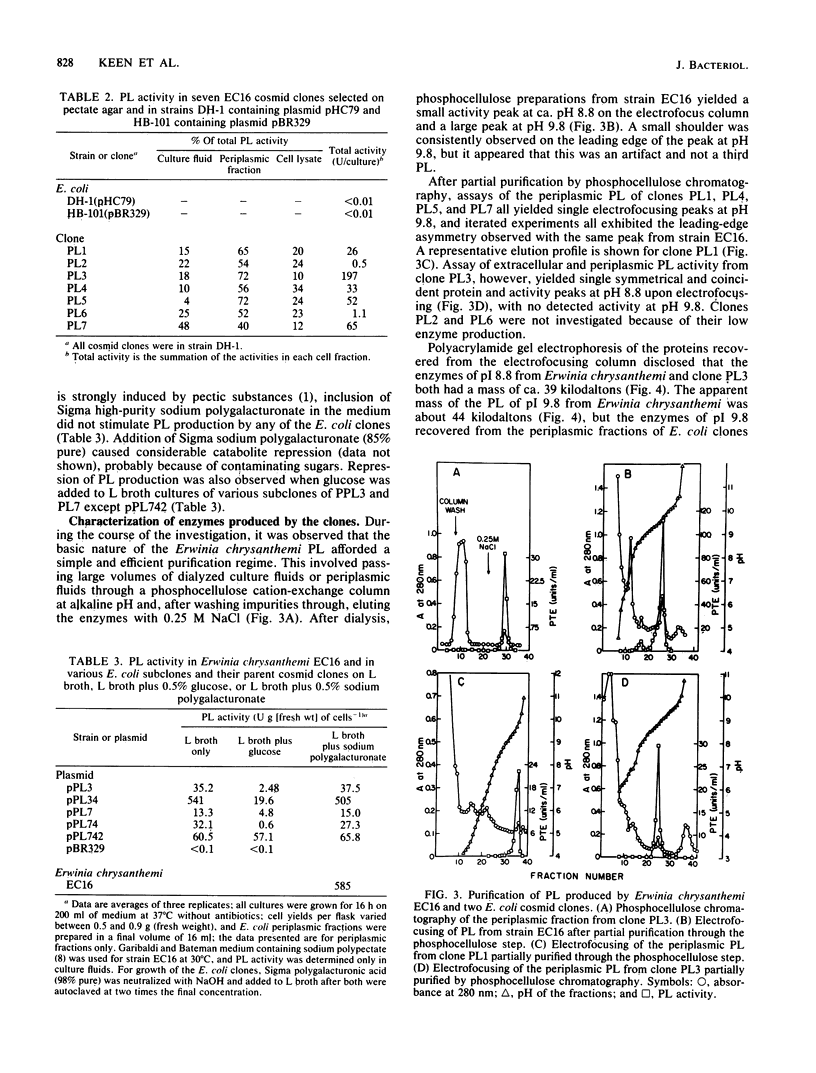
A genomic library of Erwinia chrysanthemi EC16 was constructed in plasmid pHC79, and seven putative pectate lyase (PL) clones in Escherichia coli were selected on pectate agar. Six of the recombinant cosmids contained a common PstI fragment of ca. 8.2 kilobases (kb). Subcloning of this fragment in either orientation into the PstI site of plasmid pBR329 resulted in E. coli transformants that produced a PL of pI 9.8 which was indistinguishable from one of two PLs produced by strain EC16. A 6.6-kilobase PstI fragment from the remaining cosmid clone caused production of an Erwinia PL of pI 8.8 when the fragment was subcloned in either orientation into plasmid pBR329 and transformed into E. coli. Selected pBR329 subclones for the 8.2- and 6.6-kilobase PstI fragments showed no similarity in their restriction maps and did not cross-hybridize. All of the E. coli cosmid clones that produced large amounts of PL also caused soft-rot of potato tubers and tuber slices, thus confirming the role of the enzymes in plant tissue maceration. The E. coli cosmid clones and plasmid pBR329 subclones produced the PLs constitutively, unlike Erwinia chrysanthemi, which made the enzymes inducibly. However, catabolite repression appeared to function in the E. coli clones, and almost all of the PL activity occurred in the periplasm and culture fluids. Thus, the Erwinia PL clones appear to contain signal peptide sequences, transcription and translation signals, and a recognition sequence for the catabolite activator protein, all of which function efficiently in E. coli.
Full text
PDF

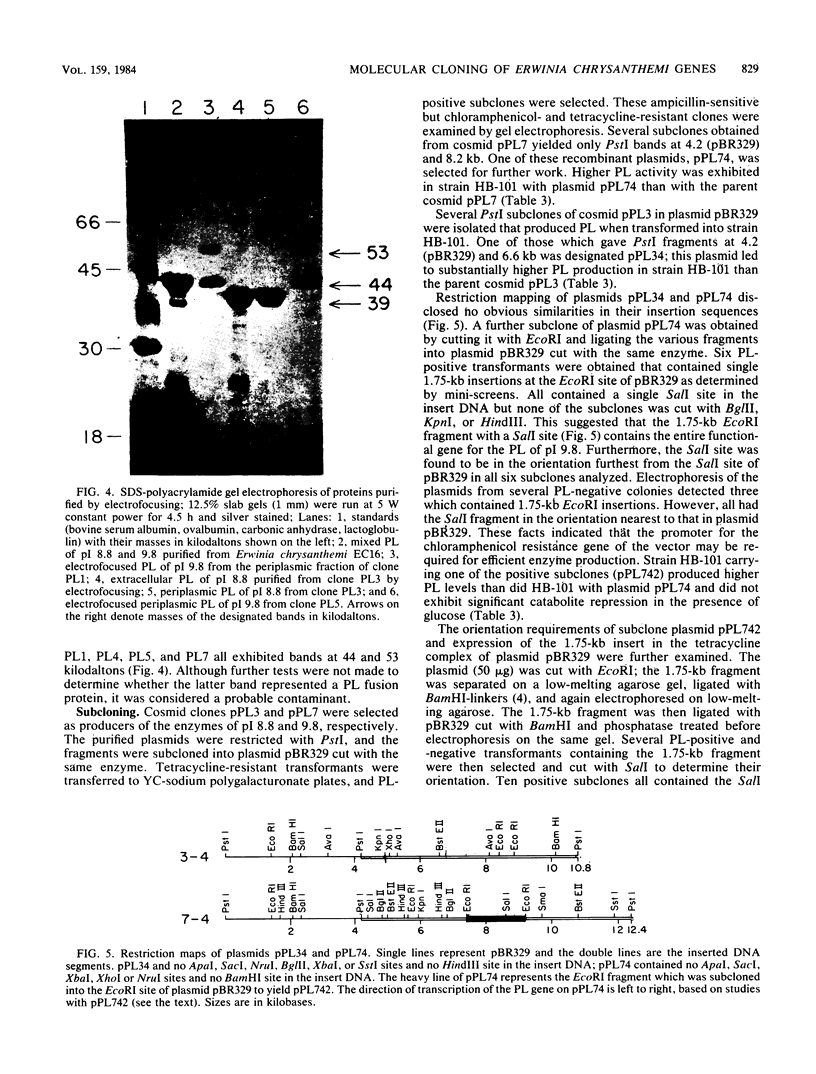


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chatterjee A. K., Buchanan G. E., Behrens M. K., Starr M. P. Synthesis and excretion of polygalacturonic acid trans-eliminase in Erwinia, Yersinia, and Klebsiella species. Can J Microbiol. 1979 Jan;25(1):94–102. doi: 10.1139/m79-014. [DOI] [PubMed] [Google Scholar]
- Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. VI. Plasmid pBR329, a new derivative of pBR328 lacking the 482-base-pair inverted duplication. Gene. 1982 Jan;17(1):79–89. doi: 10.1016/0378-1119(82)90103-2. [DOI] [PubMed] [Google Scholar]
- Crouse G. F., Frischauf A., Lehrach H. An integrated and simplified approach to cloning into plasmids and single-stranded phages. Methods Enzymol. 1983;101:78–89. doi: 10.1016/0076-6879(83)01006-x. [DOI] [PubMed] [Google Scholar]
- Davis K. R., Lyon G. D., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XXV. Endopolygalacturonic Acid Lyase from Erwinia carotovora Elicits Phytoalexin Accumulation by Releasing Plant Cell Wall Fragments. Plant Physiol. 1984 Jan;74(1):52–60. doi: 10.1104/pp.74.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg A. J., Holmes D. S. A note on the use of CsCl centrifugation to purify bacterial plasmids prepared by the rapid boiling method. Anal Biochem. 1982 Dec;127(2):434–434. doi: 10.1016/0003-2697(82)90199-3. [DOI] [PubMed] [Google Scholar]
- Gardner J. M., Kado C. I. Polygalacturonic acid trans-eliminase in the osmotic shock fluid of Erwinia rubrifaciens: characterization of the purified enzyme and its effect on plant cells. J Bacteriol. 1976 Jul;127(1):451–460. doi: 10.1128/jb.127.1.451-460.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Hohn B., Murray K. Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3259–3263. doi: 10.1073/pnas.74.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen N. T., Yoshikawa M. beta-1,3-Endoglucanase from Soybean Releases Elicitor-Active Carbohydrates from Fungus Cell Walls. Plant Physiol. 1983 Mar;71(3):460–465. doi: 10.1104/pp.71.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Morrison D. A. Transformation in Escherichia coli: cryogenic preservation of competent cells. J Bacteriol. 1977 Oct;132(1):349–351. doi: 10.1128/jb.132.1.349-351.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaley R. F. Molecular biology of extracellular enzymes. Adv Appl Microbiol. 1979;25:37–55. doi: 10.1016/s0065-2164(08)70145-x. [DOI] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]
- Zucker M., Hankin L. Regulation of pectate lyase synthesis in Pseudomonas fluorescens and Erwinia carotovora. J Bacteriol. 1970 Oct;104(1):13–18. doi: 10.1128/jb.104.1.13-18.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]