Abstract
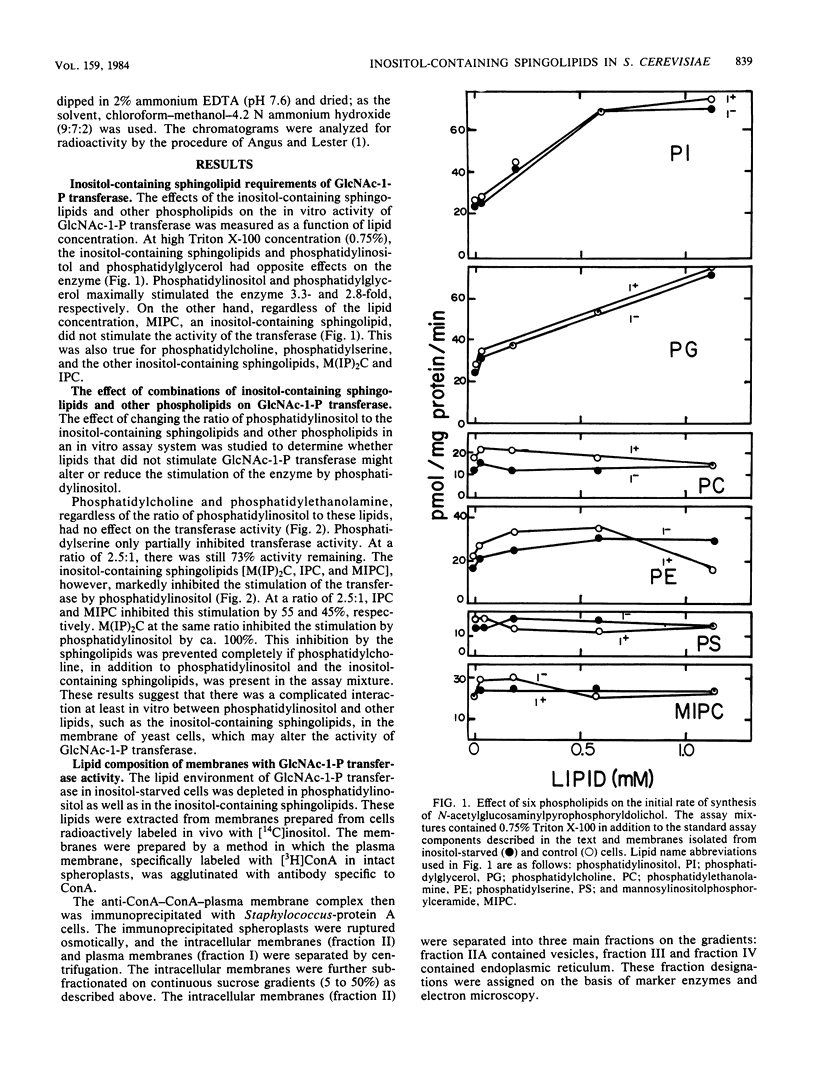
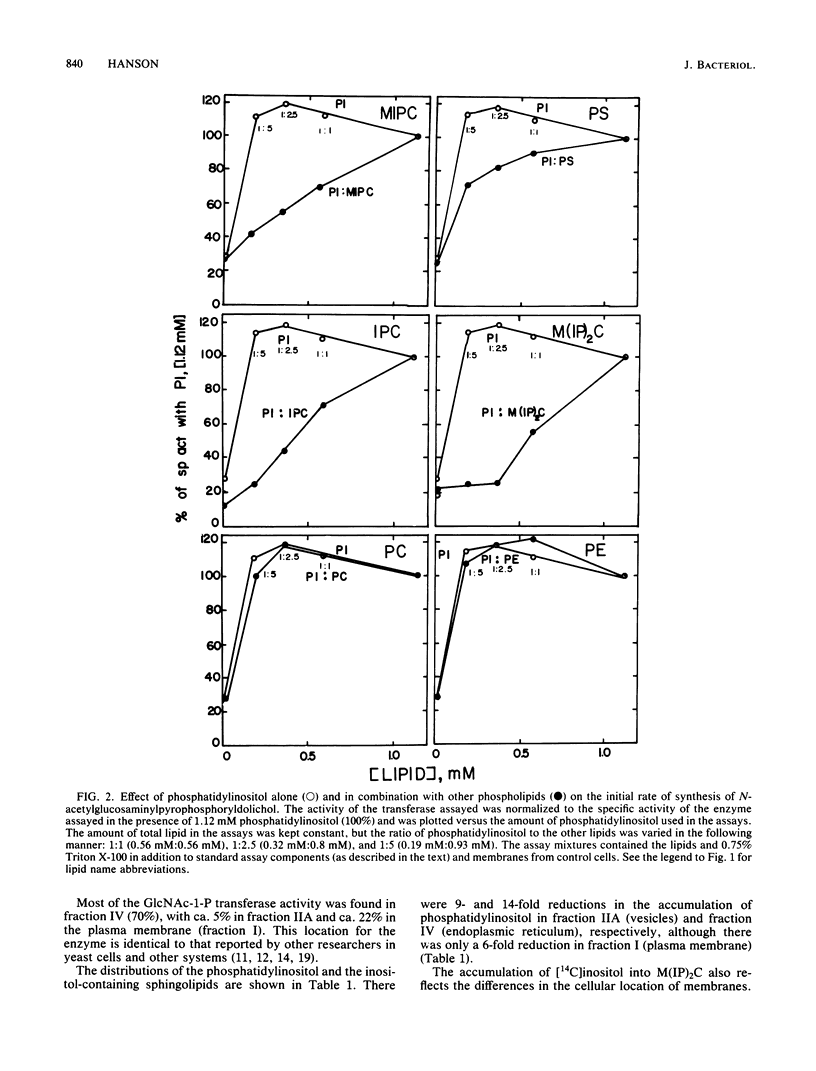
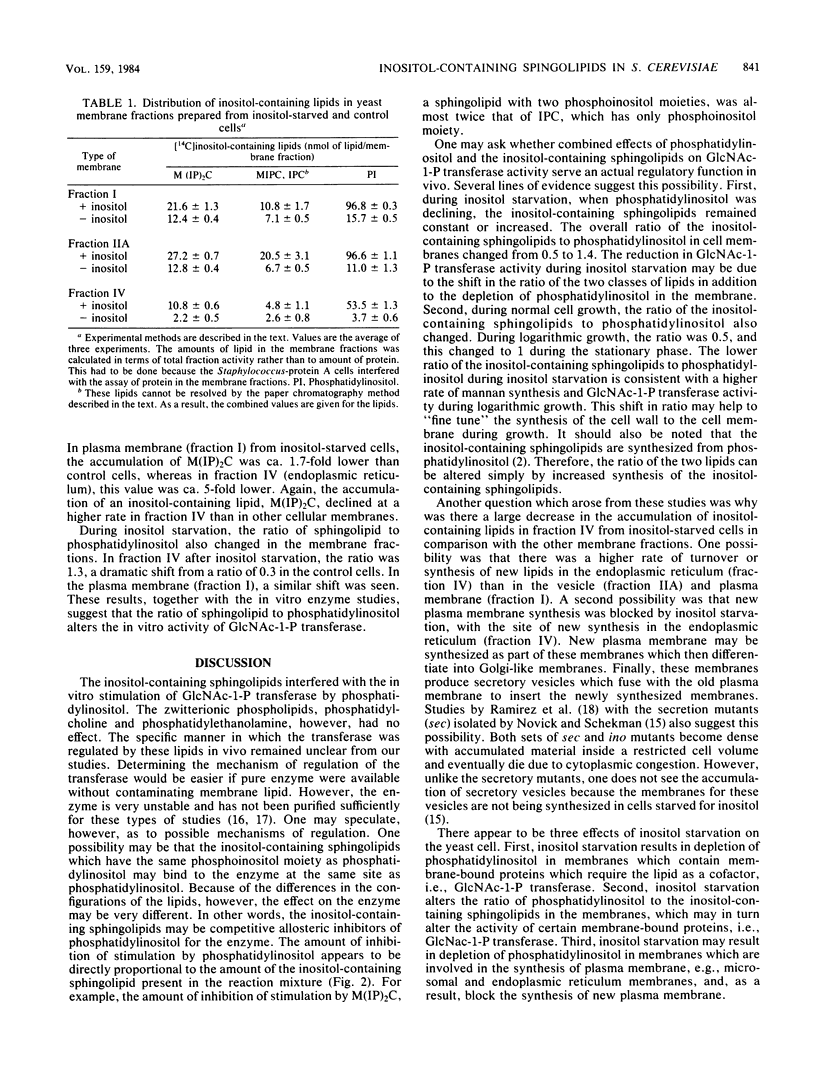
The in vitro lipid requirements of UDP-N-acetylglucosamine-dolichol phosphate N-acetylglucosamine-1-phosphotransferase for the inositol-containing sphingolipids from Saccharomyces cerevisiae were characterized in terms of concentration and specificity. The effects of combinations of lipids, especially phosphatidylinositol and the inositol-containing sphingolipids, were also tested on the transferase. Phosphatidylinositol and phosphatidylglycerol stimulated the enzyme 3.3- and 2.8-fold, respectively. The inositol-containing sphingolipids, phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine did not stimulate the activity of the transferase. Phosphatidylcholine and phosphatidylethanolamine in combination with phosphatidylinositol had no effect on the transferase activity; however, the inositol-containing sphingolipids markedly inhibited the stimulation of the transferase by phosphatidylinositol. This inhibition by the sphingolipids was prevented if phosphatidylcholine, in addition to the other lipids, was present in the assay mixture. In addition, changes due to inositol starvation in the in vivo membrane lipid environment, i.e., phosphatidylinositol and the inositol-containing sphingolipids, were analyzed to determine whether they corresponded to the observed in vitro effects. Three hours after the beginning of inositol starvation, there were 9- and 14-fold reductions in the accumulation of phosphatidylinositol in membrane fractions IIA (vesicles) and IV (endoplasmic reticulum), respectively, although there was only a 6-fold reduction in membrane fraction I (plasma membrane). The accumulation of [14C]inositol into inositol-containing sphingolipids also reflected the differences in the cellular location of membranes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus W. W., Lester R. L. Turnover of inositol and phosphorus containing lipids in Saccharomyces cerevisiae; extracellular accumulation of glycerophosphorylinositol derived from phosphatidylinositol. Arch Biochem Biophys. 1972 Aug;151(2):483–495. doi: 10.1016/0003-9861(72)90525-5. [DOI] [PubMed] [Google Scholar]
- Becker G. W., Lester R. L. Biosynthesis of phosphoinositol-containing sphingolipids from phosphatidylinositol by a membrane preparation from Saccharomyces cerevisiae. J Bacteriol. 1980 Jun;142(3):747–754. doi: 10.1128/jb.142.3.747-754.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson M. R., Donahue T. F., Henry S. A. Control of inositol biosynthesis in Saccharomyces cerevisiae; inositol-phosphate synthetase mutants. J Bacteriol. 1976 Apr;126(1):243–250. doi: 10.1128/jb.126.1.243-250.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson M. R., Henry S. A. Inositol-requiring mutants of Saccharomyces cerevisiae. Genetics. 1975 May;80(1):23–40. doi: 10.1093/genetics/80.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field C., Schekman R. Localized secretion of acid phosphatase reflects the pattern of cell surface growth in Saccharomyces cerevisiae. J Cell Biol. 1980 Jul;86(1):123–128. doi: 10.1083/jcb.86.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson B. A., Lester R. L. Effect of inositol starvation on the in vitro syntheses of mannan and N-acetylglucosaminylpyrophosphoryldolichol in Saccharomyces cerevisiae. J Bacteriol. 1982 Jul;151(1):334–342. doi: 10.1128/jb.151.1.334-342.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson B. A., Lester R. L. Effects of inositol starvation on phospholipid and glycan syntheses in Saccharomyces cerevisiae. J Bacteriol. 1980 Apr;142(1):79–89. doi: 10.1128/jb.142.1.79-89.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Atkinson K. D., Kolat A. I., Culbertson M. R. Growth and metabolism of inositol-starved Saccharomyces cerevisiae. J Bacteriol. 1977 Apr;130(1):472–484. doi: 10.1128/jb.130.1.472-484.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Donahue T. F., Culbertson M. R. Selection of spontaneous mutants by inositol starvation in yeast. Mol Gen Genet. 1975 Dec 30;143(1):5–11. doi: 10.1007/BF00269415. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehle L., Bauer F., Tanner W. The formation of glycosidic bonds in yeast glycoproteins. Intracellular localisation of the reactions. Arch Microbiol. 1977 Jul 26;114(1):77–81. doi: 10.1007/BF00429634. [DOI] [PubMed] [Google Scholar]
- Lehle L., Tanner W. The specific site of tunicamycin inhibition in the formation of dolichol-bound N-acetylglucosamine derivatives. FEBS Lett. 1976 Nov 15;72(1):167–170. doi: 10.1016/0014-5793(76)80922-2. [DOI] [PubMed] [Google Scholar]
- Marriott M., Tanner W. Localization of dolichyl phosphate- and pyrophosphate-dependent glycosyl transfer reactions in Saccharomyces cerevisiae. J Bacteriol. 1979 Aug;139(2):566–572. doi: 10.1128/jb.139.2.566-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouhar P. L., Bretthauer R. K. A phospholipid requirement for dolichol pyrophosphate N-acetylglucosamine synthesis in phospholipase A2-treated rat lung microsomes. J Biol Chem. 1982 Aug 10;257(15):8907–8911. [PubMed] [Google Scholar]
- Plouhar P. L., Bretthauer R. K. Enhancement by acidic phospholipids of the rate of synthesis of N-acetylglucosaminylpyrophosphoryldolichol from dolichol phosphate in rat lung membranes. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1186–1193. doi: 10.1016/0006-291x(79)91162-8. [DOI] [PubMed] [Google Scholar]
- Ramirez R. M., Ishida-Schick T., Krilowicz B. L., Leish B. A., Atkinson K. D. Plasma membrane expansion terminates in Saccharomyces cerevisiae secretion-defective mutants while phospholipid synthesis continues. J Bacteriol. 1983 Jun;154(3):1276–1283. doi: 10.1128/jb.154.3.1276-1283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuvers F., Habets-Willems C., Reinking A., Boer P. Glycolipid intermediates involved in the transfer of N-acetylglucosamine to endogenous proteins in a yeast membrane preparation. Biochim Biophys Acta. 1977 Mar 25;486(3):541–552. doi: 10.1016/0005-2760(77)90104-7. [DOI] [PubMed] [Google Scholar]
- Smith S. W., Lester R. L. Inositol phosphorylceramide, a novel substance and the chief member of a major group of yeast sphingolipids containing a single inositol phosphate. J Biol Chem. 1974 Jun 10;249(11):3395–3405. [PubMed] [Google Scholar]
- Waechter C. J., Harford J. B. Evidence for the enzymatic transfer of N-acetylglucosamine from UDP--N-acetylglucosamine into dolichol derivative and glycoproteins by calf brain membranes. Arch Biochem Biophys. 1977 May;181(1):185–198. doi: 10.1016/0003-9861(77)90497-0. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lester R. L. Regulation of phosphatidylcholine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1971 Mar;105(3):837–843. doi: 10.1128/jb.105.3.837-843.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



