Abstract
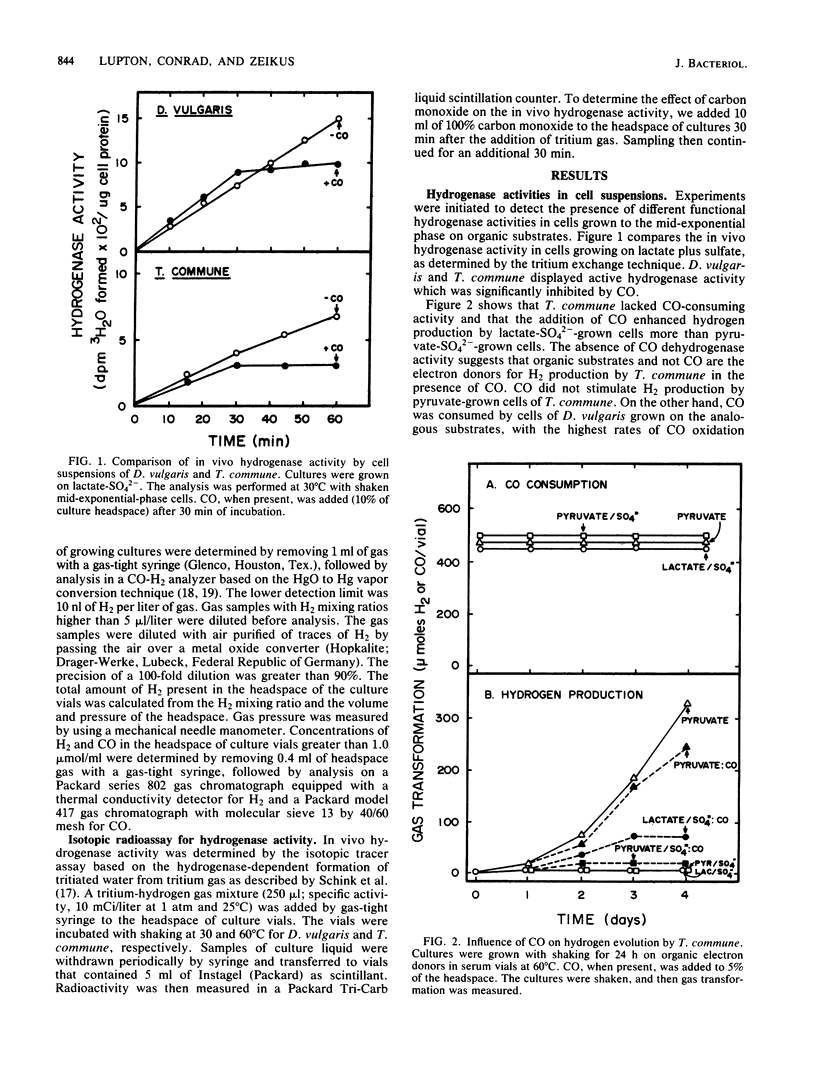
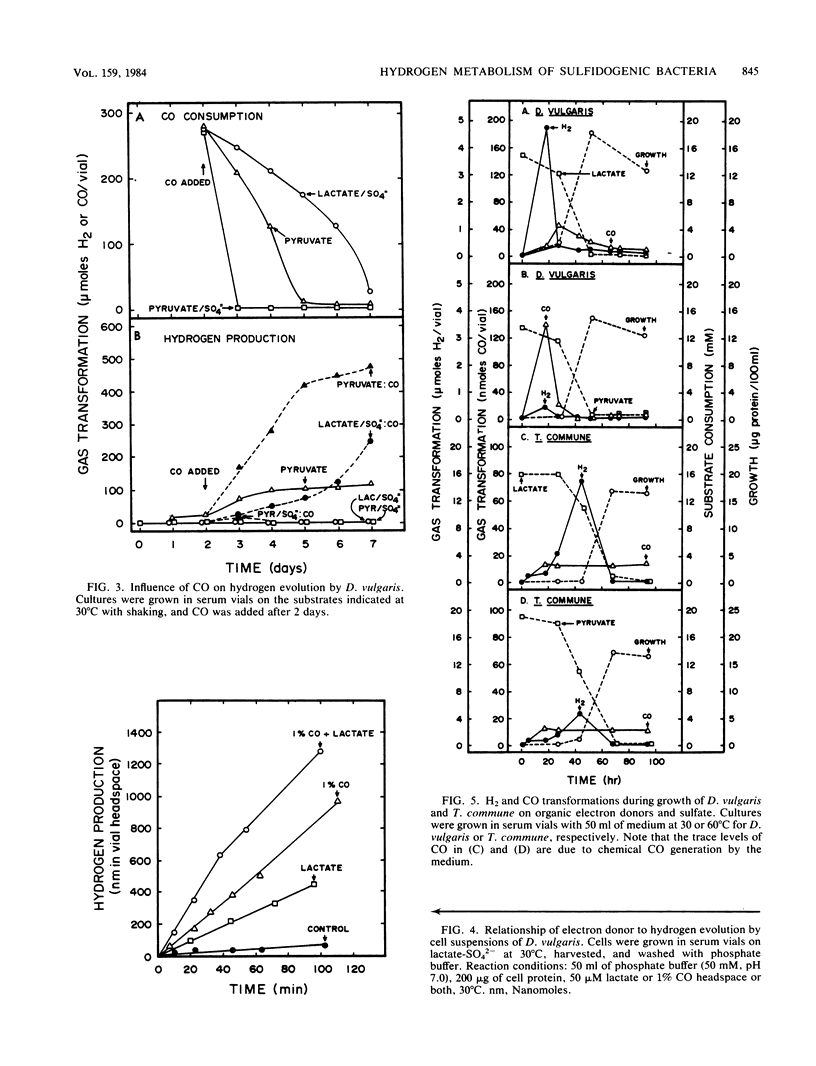
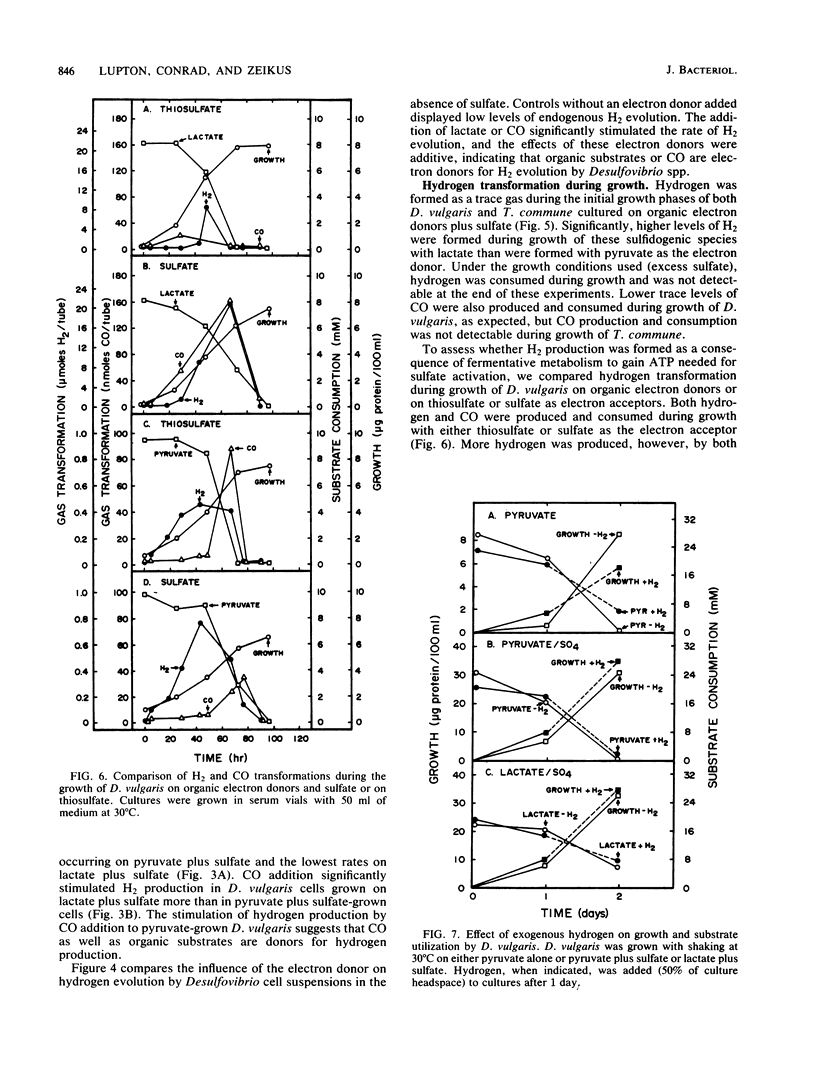
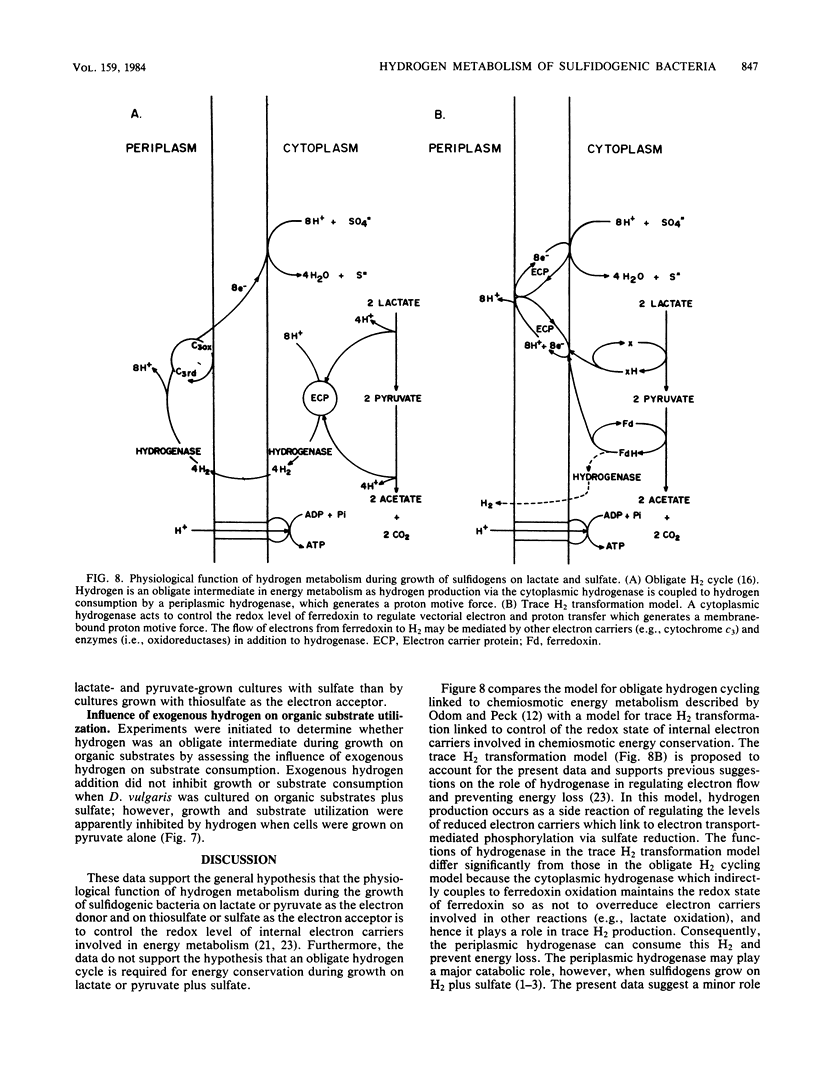
Desulfovibrio vulgaris Madison and Thermodesulfobacterium commune contained functionally distinct hydrogenase activities, one which exchanged 3H2 into 3H2O and was inhibited by carbon monoxide and a second activity which produced H2 in the presence of CO. Cell suspensions of D. vulgaris used either lactate, pyruvate, or CO as the electron donor for H2 production in the absence of sulfate. Both sulfidogenic species produced and consumed hydrogen as a trace gas during growth on lactate or pyruvate as electron donors and on thiosulfate or sulfate as electron acceptors. Higher initial levels of hydrogen were detected during growth on lactate-sulfate than on pyruvate-sulfate. D. vulgaris but not T. commune also produced and then consumed CO during growth on organic electron donors and sulfate or thiosulfate. High partial pressures of exogenous H2 inhibited growth and substrate consumption when D. vulgaris was cultured on pyruvate alone but not when it was metabolizing pyruvate plus sulfate or lactate plus sulfate. The data are discussed in relation to supporting two different models for the physiological function of H2 metabolism during growth of sulfidogenic bacteria on organic electron donors plus sulfate. A trace H2 transformation model is proposed for control of redox processes during growth on either pyruvate or lactate plus sulfate, and an obligate H2 cycling model is proposed for chemiosmotic energy coupling during growth on CO plus sulfate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badziong W., Thauer R. K. Growth yields and growth rates of Desulfovibrio vulgaris (Marburg) growing on hydrogen plus sulfate and hydrogen plus thiosulfate as the sole energy sources. Arch Microbiol. 1978 May 30;117(2):209–214. doi: 10.1007/BF00402310. [DOI] [PubMed] [Google Scholar]
- Badziong W., Thauer R. K., Zeikus J. G. Isolation and characterization of Desulfovibrio growing on hydrogen plus sulfate as the sole energy source. Arch Microbiol. 1978 Jan 23;116(1):41–49. doi: 10.1007/BF00408732. [DOI] [PubMed] [Google Scholar]
- Boone D. R., Bryant M. P. Propionate-Degrading Bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from Methanogenic Ecosystems. Appl Environ Microbiol. 1980 Sep;40(3):626–632. doi: 10.1128/aem.40.3.626-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Campbell L. L., Reddy C. A., Crabill M. R. Growth of desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria. Appl Environ Microbiol. 1977 May;33(5):1162–1169. doi: 10.1128/aem.33.5.1162-1169.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchikian E. C., Zeikus J. G. Characterization of a new type of dissimilatory sulfite reductase present in Thermodesulfobacterium commune. J Bacteriol. 1983 Mar;153(3):1211–1220. doi: 10.1128/jb.153.3.1211-1220.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin S. M., Glick B. R., Martin W. G. Factors affecting the production of hydrogenase by Desulfovibrio desulfuricans. Can J Microbiol. 1980 Oct;26(10):1209–1213. doi: 10.1139/m80-202. [DOI] [PubMed] [Google Scholar]
- Ogata M., Arihara K., Yagi T. D-lactate dehydrogenase of Desulfovibrio vulgaris. J Biochem. 1981 May;89(5):1423–1431. doi: 10.1093/oxfordjournals.jbchem.a133334. [DOI] [PubMed] [Google Scholar]
- Peck H. D., Jr, Odom M. Anaerobic fermentations of cellulose to methane. Basic Life Sci. 1981;18:375–395. doi: 10.1007/978-1-4684-3980-9_22. [DOI] [PubMed] [Google Scholar]
- Schink B., Lupton F. S., Zeikus J. G. Radioassay for hydrogenase activity in viable cells and documentation of aerobic hydrogen-consuming bacteria living in extreme environments. Appl Environ Microbiol. 1983 May;45(5):1491–1500. doi: 10.1128/aem.45.5.1491-1500.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traore A. S., Fardeau M. L., Hatchikian C. E., Le Gall J., Belaich J. P. Energetics of Growth of a Defined Mixed Culture of Desulfovibrio vulgaris and Methanosarcina barkeri: Interspecies Hydrogen Transfer in Batch and Continuous Cultures. Appl Environ Microbiol. 1983 Nov;46(5):1152–1156. doi: 10.1128/aem.46.5.1152-1156.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traore A. S., Hatchikian C. E., Belaich J. P., Le Gall J. Microcalorimetric studies of the growth of sulfate-reducing bacteria: energetics of Desulfovibrio vulgaris growth. J Bacteriol. 1981 Jan;145(1):191–199. doi: 10.1128/jb.145.1.191-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traore A. S., Hatchikian C. E., Le Gall J., Belaich J. P. Microcalorimetric studies of the growth of sulfate-reducing bacteria: comparison of the growth parameters of some Desulfovibrio species. J Bacteriol. 1982 Feb;149(2):606–611. doi: 10.1128/jb.149.2.606-611.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. Metabolism of one-carbon compounds by chemotrophic anaerobes. Adv Microb Physiol. 1983;24:215–299. doi: 10.1016/s0065-2911(08)60387-2. [DOI] [PubMed] [Google Scholar]
- van der Westen H. M., Mayhew S. G., Veeger C. Separation of hydrogenase from intact cells of Desulfovibrio vulgaris. Purification and properties. FEBS Lett. 1978 Feb 1;86(1):122–126. doi: 10.1016/0014-5793(78)80112-4. [DOI] [PubMed] [Google Scholar]



