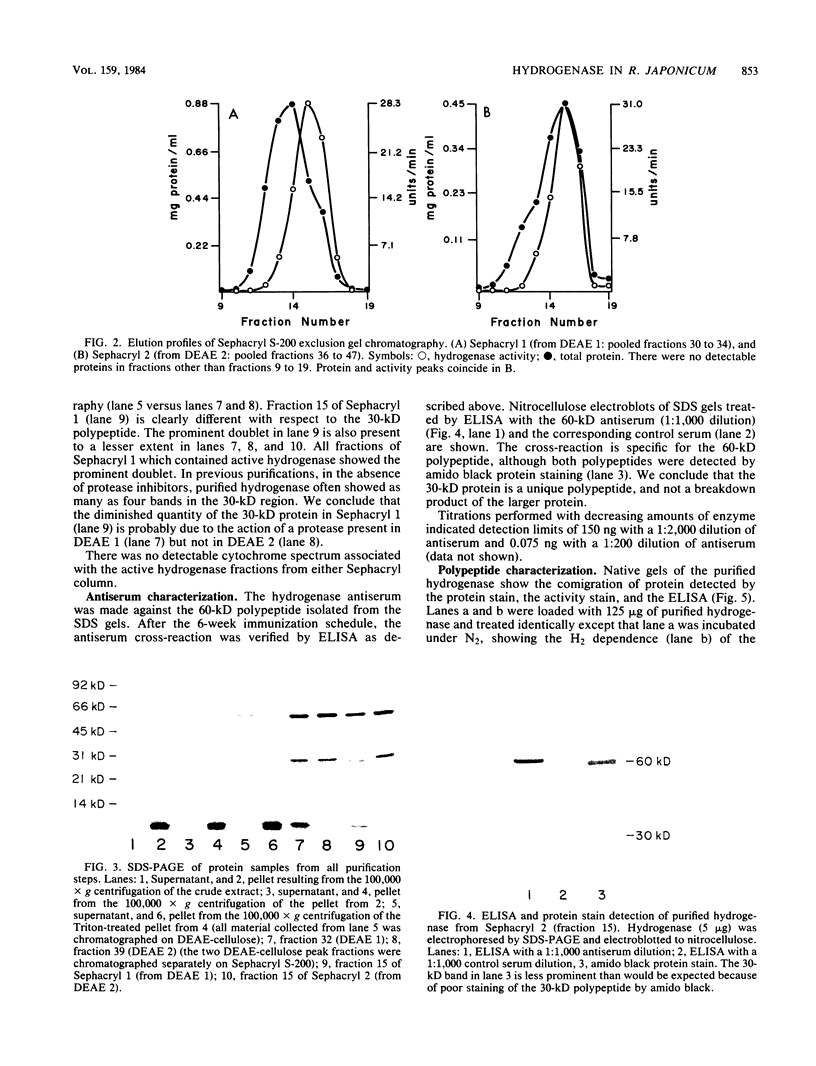
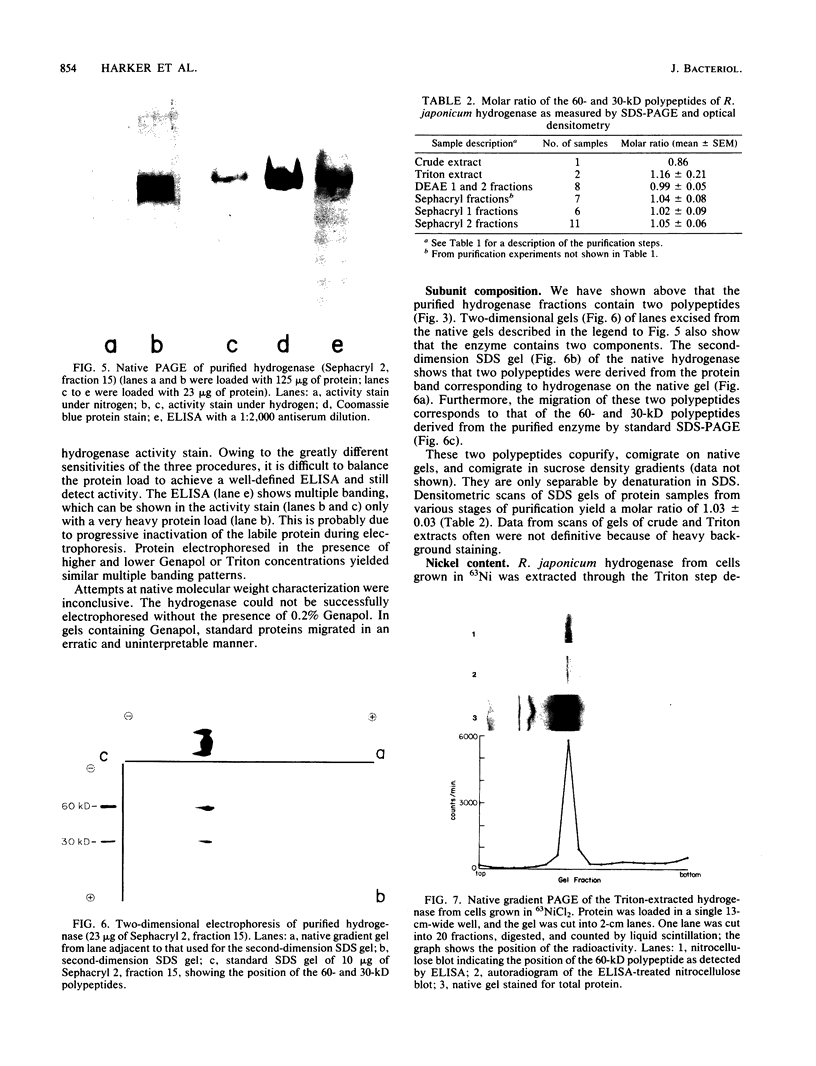
Abstract
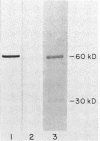
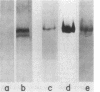
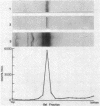
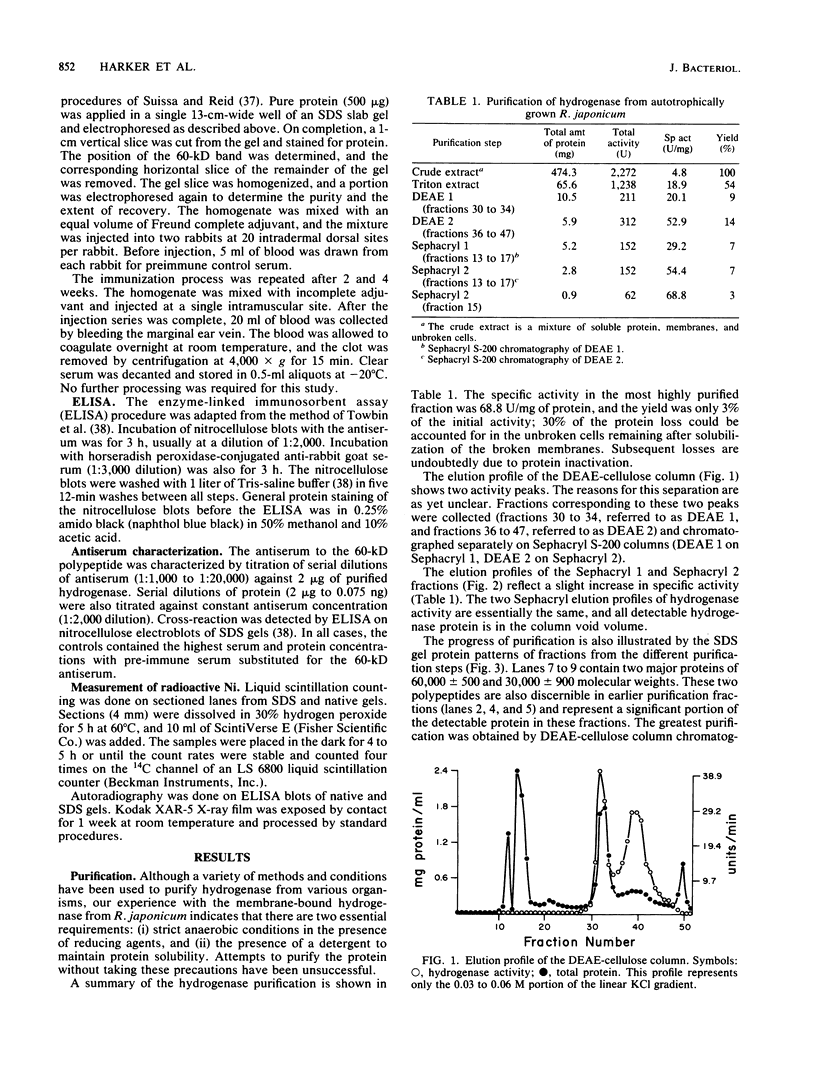
The uptake hydrogenase of chemolithotrophically grown Rhizobium japonicum was purified to apparent homogeneity with a final specific activity of 69 mumol of H2 oxidized per min per mg of protein. The procedure included Triton extraction of broken membranes and DEAE-cellulose and Sephacryl S-200 chromatographies. The purified protein contained two polypeptides separable only by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. They comigrated on native polyacrylamide gels and sucrose density gradients. The molecular weights were ca. 60,000 and 30,000. Densitometric scans of the sodium dodecyl sulfate gels indicated a molar ratio of 1.03 +/- 0.03. Antiserum was developed against the 60-kilodalton polypeptide for use in hydrogenase detection by an enzyme-linked immunosorbent assay. The antiserum did not cross-react with the 30-kilodalton polypeptide. Native gel electrophoresis of Triton-extracted cells grown in the presence of 63Ni showed comigration of the hydrogenase and radioactive Ni.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W., Hall D. O. Properties of the solubilized membrane-bound hydrogenase from the photosynthetic bacterium Rhodospirillum rubrum. Arch Biochem Biophys. 1979 Jul;195(2):288–299. doi: 10.1016/0003-9861(79)90355-2. [DOI] [PubMed] [Google Scholar]
- Adams M. W., Hall D. O. Purification of the membrane-bound hydrogenase of Escherichia coli. Biochem J. 1979 Oct 1;183(1):11–22. doi: 10.1042/bj1830011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M. W., Mortenson L. E., Chen J. S. Hydrogenase. Biochim Biophys Acta. 1980 Dec;594(2-3):105–176. doi: 10.1016/0304-4173(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Arp D. J., Burris R. H. Purification and properties of the particulate hydrogenase from the bacteroids of soybean root nodules. Biochim Biophys Acta. 1979 Oct 11;570(2):221–230. doi: 10.1016/0005-2744(79)90142-6. [DOI] [PubMed] [Google Scholar]
- BARTHA R., ORDAL E. J. NICKEL-DEPENDENT CHEMOLITHOTROPHIC GROWTH OF TWO HYDROGENOMONAS STRAINS. J Bacteriol. 1965 Apr;89:1015–1019. doi: 10.1128/jb.89.4.1015-1019.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen J. S., Mortenson L. E. Purification and properties of hydrogenase from Clostridium pasteurianum W5. Biochim Biophys Acta. 1974 Dec 18;371(2):283–298. doi: 10.1016/0005-2795(74)90025-7. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in legume root nodule bacteroids: occurrence and properties. Arch Mikrobiol. 1972;85(3):193–201. doi: 10.1007/BF00408844. [DOI] [PubMed] [Google Scholar]
- Eisbrenner G., Evans H. J. Spectral evidence for a component involved in hydrogen metabolism of soybean nodule bacteroids. Plant Physiol. 1982 Dec;70(6):1667–1672. doi: 10.1104/pp.70.6.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Heine E., Finck A., Friedrich C. G. Nickel requirement for active hydrogenase formation in Alcaligenes eutrophus. J Bacteriol. 1981 Mar;145(3):1144–1149. doi: 10.1128/jb.145.3.1144-1149.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich C. G., Schneider K., Friedrich B. Nickel in the catalytically active hydrogenase of Alcaligenes eutrophus. J Bacteriol. 1982 Oct;152(1):42–48. doi: 10.1128/jb.152.1.42-48.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlitz P. H., Krasna A. I. Structural and catalytic properties of hydrogenase from Chromatium. Biochemistry. 1975 Jun 17;14(12):2561–2568. doi: 10.1021/bi00683a001. [DOI] [PubMed] [Google Scholar]
- Haugland R. A., Hanus F. J., Cantrell M. A., Evans H. J. Rapid Colony Screening Method for Identifying Hydrogenase Activity in Rhizobium japonicum. Appl Environ Microbiol. 1983 Mar;45(3):892–897. doi: 10.1128/aem.45.3.892-897.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuno T., Kaplan N. O., Kamen M. D. Chromatium hydrogenase. Proc Natl Acad Sci U S A. 1977 Mar;74(3):861–863. doi: 10.1073/pnas.74.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucas R. V., Hanus F. J., Russell S. A., Evans H. J. Nickel: A micronutrient element for hydrogen-dependent growth of Rhizobium japonicum and for expression of urease activity in soybean leaves. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2253–2257. doi: 10.1073/pnas.80.8.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeGall J., Ljungdahl P. O., Moura I., Peck H. D., Jr, Xavier A. V., Moura J. J., Teixera M., Huynh B. H., DerVartanian D. V. The presence of redox-sensitive nickel in the periplasmic hydrogenase from Desulfovibrio gigas. Biochem Biophys Res Commun. 1982 May 31;106(2):610–616. doi: 10.1016/0006-291x(82)91154-8. [DOI] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Involvement of cytochromes and a flavoprotein in hydrogen oxidation in Rhizobium japonicum bacteroids. J Bacteriol. 1983 Aug;155(2):481–487. doi: 10.1128/jb.155.2.481-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Repaske R., Mayer R. Dense autotrophic cultures of Alcaligenes eutrophus. Appl Environ Microbiol. 1976 Oct;32(4):592–597. doi: 10.1128/aem.32.4.592-597.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske R., Repaske A. C. Quantitative requirements for exponential growth of Alcaligenes eutrophus. Appl Environ Microbiol. 1976 Oct;32(4):585–591. doi: 10.1128/aem.32.4.585-591.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. The membrane-bound hydrogenase of Alcaligenes eutrophus. I. Solubilization, purification, and biochemical properties. Biochim Biophys Acta. 1979 Apr 12;567(2):315–324. doi: 10.1016/0005-2744(79)90117-7. [DOI] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G. Purification and properties of soluble hydrogenase from Alcaligenes eutrophus H 16. Biochim Biophys Acta. 1976 Nov 8;452(1):66–80. doi: 10.1016/0005-2744(76)90058-9. [DOI] [PubMed] [Google Scholar]
- Schoenmaker G. S., Oltmann L. F., Stouthamer A. H. Purification and properties of the membrane-bound hydrogenase from Proteus mirabilis. Biochim Biophys Acta. 1979 Apr 12;567(2):511–521. doi: 10.1016/0005-2744(79)90137-2. [DOI] [PubMed] [Google Scholar]
- Sim E., Vignais P. M. Comparison of the membrane-bound and detergent-solubilised hydrogenase from paracoccus denitrificans. Isolation of the hydrogenase. Biochim Biophys Acta. 1979 Sep 12;570(1):43–55. doi: 10.1016/0005-2744(79)90199-2. [DOI] [PubMed] [Google Scholar]
- Suissa M., Reid G. A. Preparation and use of antibodies against insoluble membrane proteins. Methods Enzymol. 1983;97:305–311. doi: 10.1016/0076-6879(83)97142-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unden G., Böcher R., Knecht J., Kröger A. Hydrogenase from Vibrio succinogenes, a nickel protein. FEBS Lett. 1982 Aug 23;145(2):230–234. doi: 10.1016/0014-5793(82)80173-7. [DOI] [PubMed] [Google Scholar]