Abstract
Klebsiella pneumoniae accumulates molybdenum during nitrogenase derepression. The molybdenum is primarily in nitrogenase component I in the form of iron-molybdenum cofactor (FeMo-co). Mutations in any of three genes (nifB, nifN, and nifE) involved in the biosynthesis of FeMo-co resulted in very low molybdenum accumulation and in a molybdenum-free nitrogenase component I. A mutant lacking both subunits of nitrogenase component I accumulated 60% of the amount of molybdenum present in the wild type. The molybdenum was in protein-bound form and behaved differently than that in the wild type with respect to electrophoretic mobility, size, and extractability by organic solvents. Two forms of molybdenum could be extracted from the protein fraction of the mutant; one of them was not detected in the wild type, and the other behaved like FeMo-co in nonaqueous gel filtration chromatography. Crude extracts of this mutant were able to complement in vitro K. pneumoniae or Azotobacter vinelandii mutants unable to produce FeMo-co. These data show that biosynthesis of FeMo-co does not require the presence of nitrogenase component I. In its absence, FeMo-co is accumulated on a different protein, presumably an intermediate in the normal FeMo-co biosynthetic pathway.
Full text
PDF
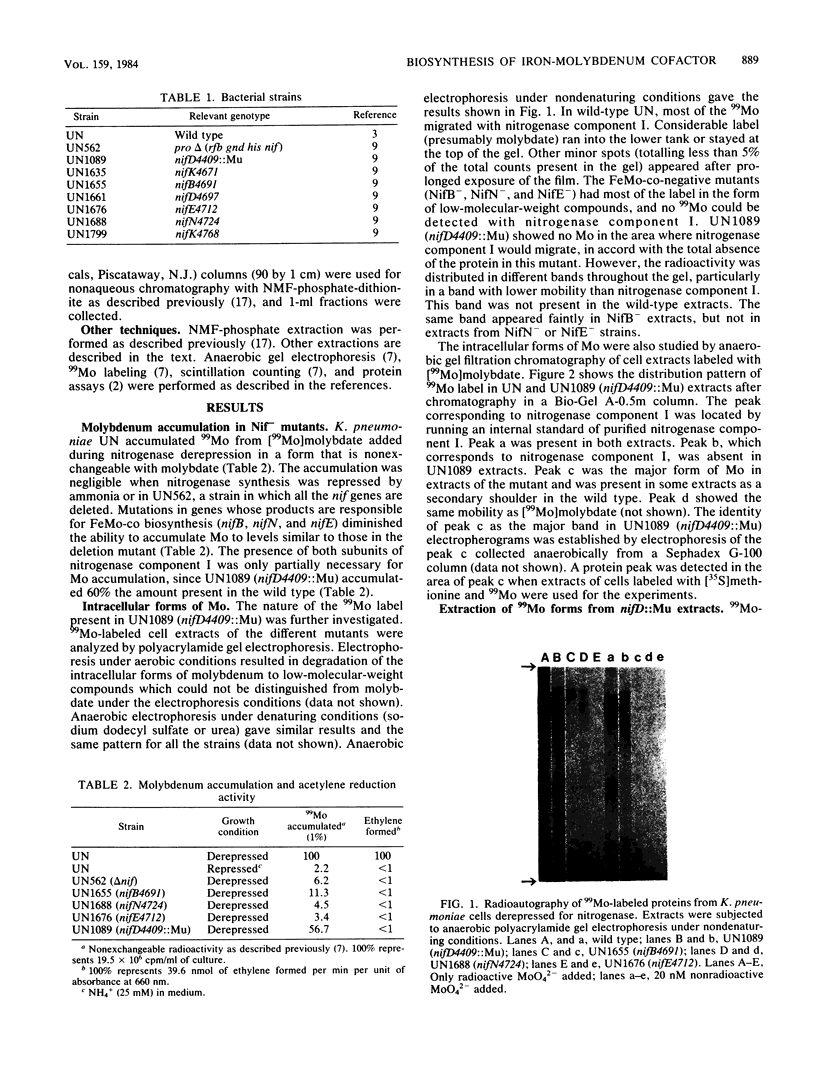
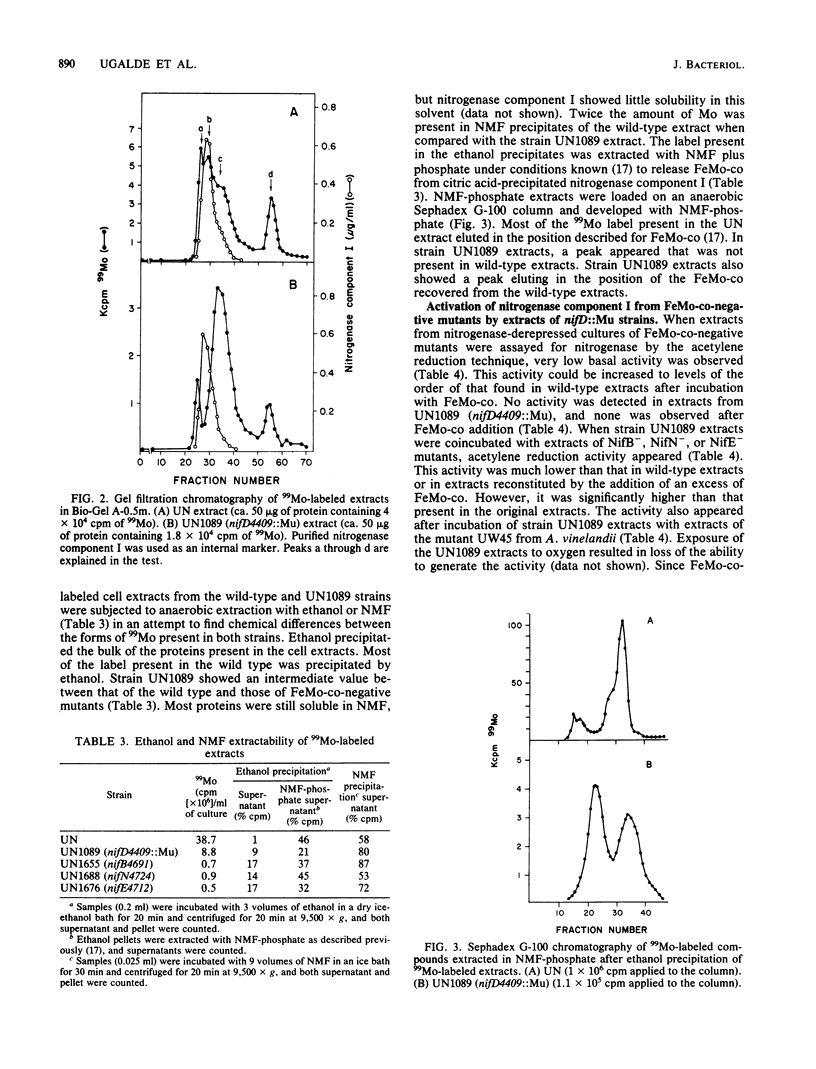
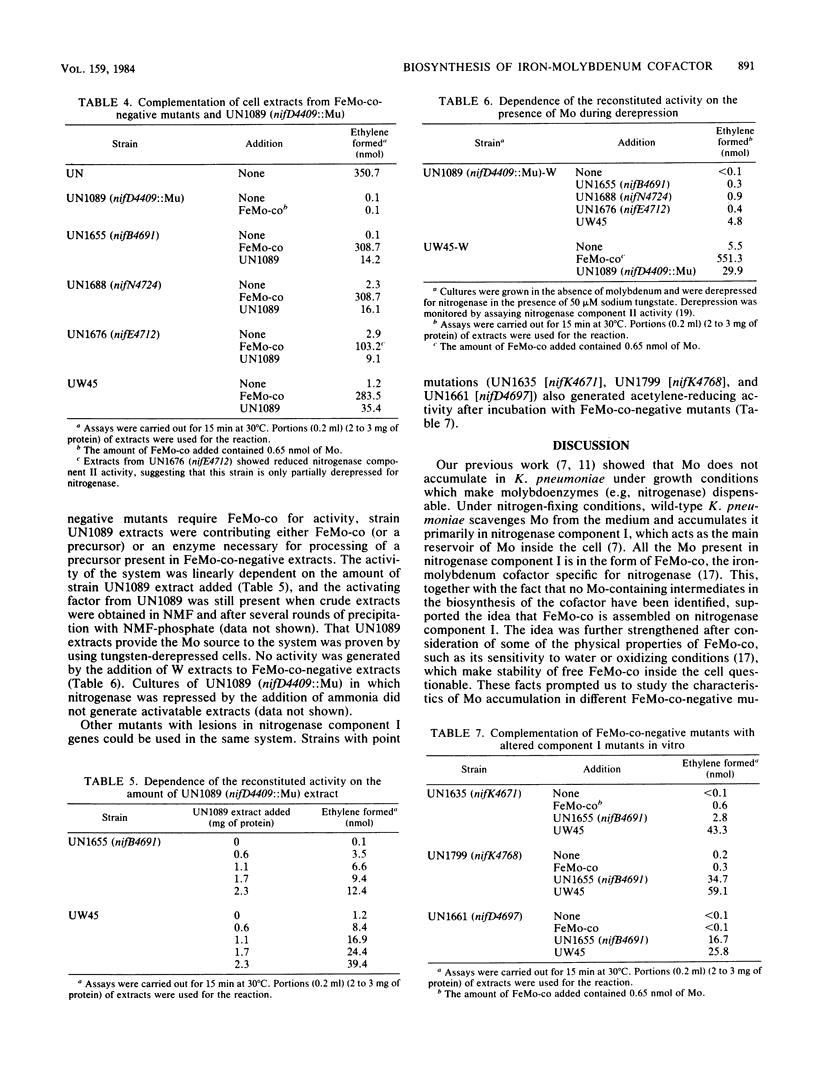


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop P. E., Brill W. J. Genetic analysis of Azotobacter vinelandii mutant strains unable to fix nitrogen. J Bacteriol. 1977 May;130(2):954–956. doi: 10.1128/jb.130.2.954-956.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brill W. J., Steiner A. L., Shah V. K. Effect of molybdenum starvation and tungsten on the synthesis of nitrogenase components in Klebsiella pneumonia. J Bacteriol. 1974 Jun;118(3):986–989. doi: 10.1128/jb.118.3.986-989.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulen W. A., LeComte J. R. The nitrogenase system from Azotobacter: two-enzyme requirement for N2 reduction, ATP-dependent H2 evolution, and ATP hydrolysis. Proc Natl Acad Sci U S A. 1966 Sep;56(3):979–986. doi: 10.1073/pnas.56.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes T. R., Smith B. E. Purification and characterization of the inactive MoFe protein (NifB-Kp1) of the nitrogenase from nifB mutants of Klebsiella pneumoniae. Biochem J. 1983 Jan 1;209(1):43–50. doi: 10.1042/bj2090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial J., Ugalde R. A., Shah V. K., Brill W. J. Role of the nifQ gene product in the incorporation of molybdenum into nitrogenase in Klebsiella pneumoniae. J Bacteriol. 1984 Apr;158(1):187–194. doi: 10.1128/jb.158.1.187-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil T., Brill W. J., Howe M. M. Bacteriophage mu-induced deletions in a plasmid containing the nif (N2 fixation) genes of Klebsiella pneumoniae. J Bacteriol. 1978 Jun;134(3):821–829. doi: 10.1128/jb.134.3.821-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil T., MacNeil D., Roberts G. P., Supiano M. A., Brill W. J. Fine-structure mapping and complementation analysis of nif (nitrogen fixation) genes in Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):253–266. doi: 10.1128/jb.136.1.253-266.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani H. H., Shah V. K., Brill W. J. Activation of inactive nitrogenase by acid-treated component I. J Bacteriol. 1974 Nov;120(2):697–701. doi: 10.1128/jb.120.2.697-701.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienkos P. T., Brill W. J. Molybdenum accumulation and storage in Klebsiella pneumoniae and Azotobacter vinelandii. J Bacteriol. 1981 Feb;145(2):743–751. doi: 10.1128/jb.145.2.743-751.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienkos P. T., Shah V. K., Brill W. J. Molybdenum cofactors from molybdoenzymes and in vitro reconstitution of nitrogenase and nitrate reductase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5468–5471. doi: 10.1073/pnas.74.12.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings J., Shah V. K., Chisnell J. R., Brill W. J., Zimmermann R., Münck E., Orme-Johnson W. H. Novel metal cluster in the iron-molybdenum cofactor of nitrogenase. Spectroscopic evidence. J Biol Chem. 1978 Feb 25;253(4):1001–1004. [PubMed] [Google Scholar]
- Roberts G. P., Brill W. J. Gene-product relationships of the nif regulon of Klebsiella pneumoniae. J Bacteriol. 1980 Oct;144(1):210–216. doi: 10.1128/jb.144.1.210-216.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P., MacNeil T., MacNeil D., Brill W. J. Regulation and characterization of protein products coded by the nif (nitrogen fixation) genes of Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):267–279. doi: 10.1128/jb.136.1.267-279.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Nitrogenase. IV. Simple method of purification to homogeneity of nitrogenase components from Azotobacter vinelandii. Biochim Biophys Acta. 1973 May 30;305(2):445–454. doi: 10.1016/0005-2728(73)90190-4. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Chisnell J. R., Brill W. J. Acetylene reduction by the iron-molybdenum cofactor from nitrogenase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):232–236. doi: 10.1016/0006-291x(78)91654-6. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Davis I. C., Gordon J. K., Orme-Johnson W. H., Brill W. J. Nitrogenase. 3. Nitrogenaseless mutants of Azotobacter vinelandii: activities, cross-reactions and EPR spectra. Biochim Biophys Acta. 1973 Jan 18;292(1):246–255. doi: 10.1016/0005-2728(73)90269-7. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Davis L. C., Brill W. J. Nitrogenase. I. Repression and derepression of the iron-molybdenum and iron proteins of nitrogenase in Azotobacter vinelandii. Biochim Biophys Acta. 1972 Feb 28;256(2):498–511. doi: 10.1016/0005-2728(72)90078-3. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Stacey G., Brill W. J. Electron transport to nitrogenase. Purification and characterization of pyruvate:flavodoxin oxidoreductase. The nifJ gene product. J Biol Chem. 1983 Oct 10;258(19):12064–12068. [PubMed] [Google Scholar]
- Shah V. K., Ugalde R. A., Imperial J., Brill W. J. Molybdenum in nitrogenase. Annu Rev Biochem. 1984;53:231–257. doi: 10.1146/annurev.bi.53.070184.001311. [DOI] [PubMed] [Google Scholar]
- Sibold L., Quiviger B., Charpin N., Paquelin A., Elmerich C. Cloning and expression of a DNA fragment carrying a his nifA fusion and the nifBQ operon from a nif constitutive mutant of Klebsiella pneumoniae. Biochimie. 1983 Jan;65(1):53–63. doi: 10.1016/s0300-9084(83)80029-7. [DOI] [PubMed] [Google Scholar]
- Strandberg G. W., Wilson P. W. Formation of the nitrogen-fixing enzyme system in Azotobacter vinelandii. Can J Microbiol. 1968 Jan;14(1):25–31. doi: 10.1139/m68-005. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Mortenson L. E. The nitrogen-fixing complex of bacteria. Biochim Biophys Acta. 1975 Mar 31;416(1):1–52. doi: 10.1016/0304-4173(75)90012-9. [DOI] [PubMed] [Google Scholar]