Abstract
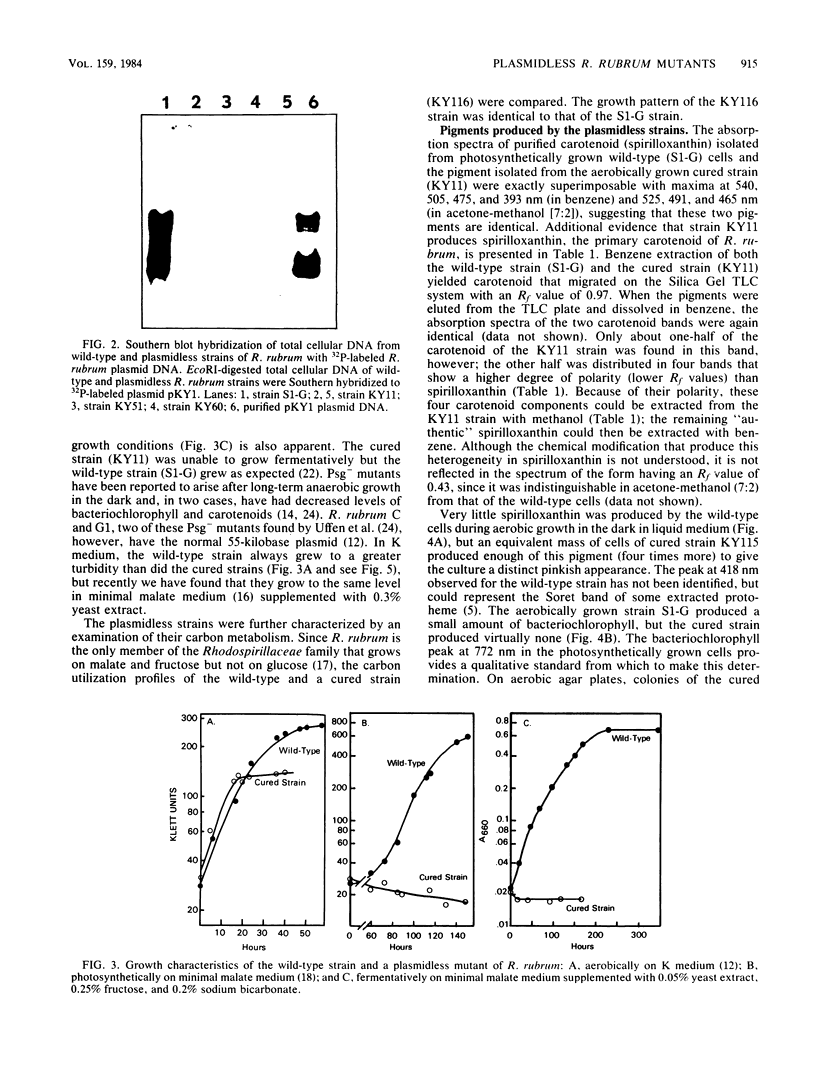
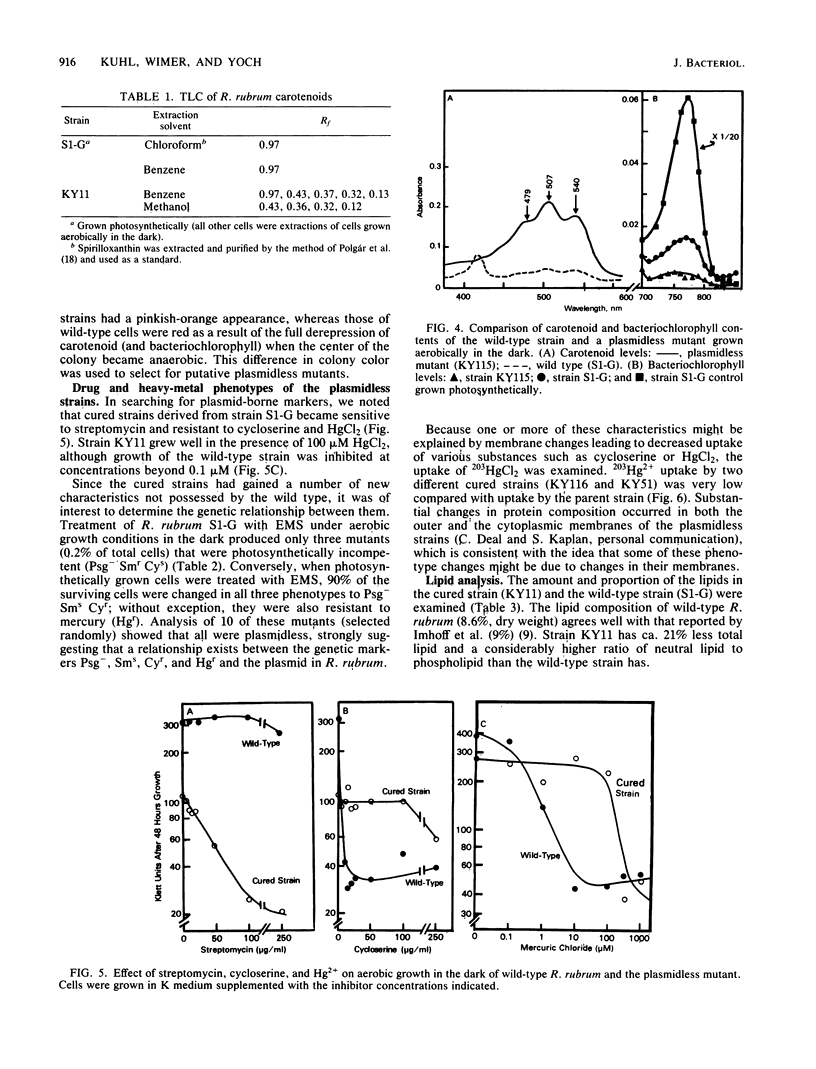
Ethyl methanesulfonate rendered a high percentage of Rhodospirillum rubrum cells plasmidless and photosynthetically incompetent (Kuhl et al., J. Bacteriol. 156:737-742, 1983). By probing restriction endonuclease-digested chromosomal DNA from these plasmidless strains with 32P-labeled R. rubrum plasmid DNA, we showed that no homology exists between the plasmid and the chromosomal DNA of the mutant. Loss of the plasmid in all the nonphotosynthetic isolates was accompanied by the synthesis of spirilloxanthin under aerobic growth conditions, resistance to cycloserine and HgCl2, and loss of ability to grow fermentatively on fructose. Changes in both the protein and lipid composition of the membranes and the impaired uptake of 203HgCl2 in the plasmidless strains (compared with the wild type) suggest either that membrane modification occurs as a result of plasmid loss, accounting for several of the acquired phenotype characteristics of the cured strains, or that both membrane modification and plasmid loss are part of the same pleiotropic mutation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biel A. J., Marrs B. L. Transcriptional regulation of several genes for bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata in response to oxygen. J Bacteriol. 1983 Nov;156(2):686–694. doi: 10.1128/jb.156.2.686-694.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derylo M., Glowacka M., Lorkiewicz Z., Russa R. Plasmid-determined alterations of Salmonella typhimurium lipopolysaccharides. Mol Gen Genet. 1975 Sep 29;140(2):175–181. doi: 10.1007/BF00329785. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gibson K. D., Niederman R. A. Characterization of two circular satellite species of deoxyribonucleic acid in Rhodopseudomonas spheroides. Arch Biochem Biophys. 1970 Dec;141(2):694–704. doi: 10.1016/0003-9861(70)90190-6. [DOI] [PubMed] [Google Scholar]
- Imhoff J. F., Kushner D. J., Kushwaha S. C., Kates M. Polar lipids in phototrophic bacteria of the Rhodospirillaceae and Chromatiaceae families. J Bacteriol. 1982 Jun;150(3):1192–1201. doi: 10.1128/jb.150.3.1192-1201.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenward M. A., Brown M. R., Hesslewood S. R., Dillon C. Influence of R-plasmid RP1 of Pseudomonas aeruginosa on cell wall composition, drug resistance, and sensitivity to cold shock. Antimicrob Agents Chemother. 1978 Mar;13(3):446–453. doi: 10.1128/aac.13.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl S. A., Nix D. W., Yoch D. C. Characterization of a Rhodospirillum rubrum plasmid: loss of photosynthetic growth in plasmidless strains. J Bacteriol. 1983 Nov;156(2):737–742. doi: 10.1128/jb.156.2.737-742.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOCHANG T. C., SWEELEY C. C. CHARACTERIZATION OF LIPIDS FROM CANINE ADRENAL GLANDS. Biochemistry. 1963 May-Jun;2:592–604. doi: 10.1021/bi00903a036. [DOI] [PubMed] [Google Scholar]
- Madigan M., Cox J. C., Gest H. Photopigments in Rhodopseudomonas capsulata cells grown anaerobically in darkness. J Bacteriol. 1982 Jun;150(3):1422–1429. doi: 10.1128/jb.150.3.1422-1429.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs B. Mobilization of the genes for photosynthesis from Rhodopseudomonas capsulata by a promiscuous plasmid. J Bacteriol. 1981 Jun;146(3):1003–1012. doi: 10.1128/jb.146.3.1003-1012.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Radin N. S. Extraction of tissue lipids with a solvent of low toxicity. Methods Enzymol. 1981;72:5–7. doi: 10.1016/s0076-6879(81)72003-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saunders V. A., Saunders J. R., Bennett P. M. Extrachromosomal deoxyribonucleic acid in wild-type and photosynthetically incompetent strains of Rhodopseudomonas spheroides. J Bacteriol. 1976 Mar;125(3):1180–1187. doi: 10.1128/jb.125.3.1180-1187.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J. E., Weaver P. F. Fermentation and anaerobic respiration by Rhodospirillum rubrum and Rhodopseudomonas capsulata. J Bacteriol. 1982 Jan;149(1):181–190. doi: 10.1128/jb.149.1.181-190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Uffen R. L., Sybesma C., Wolfe R. S. Mutants of Rhodospirrillum rubrum obtained after long-term anaerobic, dark growth. J Bacteriol. 1971 Dec;108(3):1348–1356. doi: 10.1128/jb.108.3.1348-1356.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]