Abstract
Aim:
To determine the clinical efficacy of two-year continuous subantimicrobial dose doxycycline (SDD; 20 mg bid) in postmenopausal, osteopenic, estrogen-deficient women on periodontal maintenance.
Material and Methods:
128 subjects were randomized to SDD (n = 64) or placebo (n = 64). Clinical measurements were performed at posterior interproximal sites at baseline and every six months during this 2-year randomized, double-blind, placebo-controlled, clinical trial with adjunctive, no-cost 3−4 month periodontal maintenance. Statistical analyses of secondary outcomes from this clinical trial used Generalized Estimating Equations in primarily intent-to-treat analyses (ITT).
Results:
For the placebo group, 3.4% of sites showed improvement in clinical attachment levels (CAL) and 2.7% had progressive loss in CAL; for the SDD group, 5.0% of sites showed improvement in CAL and 2.2% had progressive loss in CAL. This difference (2.1% of sites) was more favorable in the SDD group than in the placebo (OR = 0.81, 95% CI: 0.67−0.97, p = 0.03) in these well-maintained patients, whereas probing depths, bleeding on probing and supragingival plaque did not differ significantly between groups (p > 0.2).
Conclusions:
Analyses of secondary outcomes of this clinical trial indicated that SDD may be of benefit in reducing progressive attachment loss in postmenopausal females; additional research is needed to confirm these findings.
Keywords: periodontitis, osteopenia, randomized clinical trial, subantimicrobial dose doxycycline, postmenopausal, periodontal maintenance, relative clinical attachment
Clinical Relevance
Scientific Rationale:
Postmenopausal estrogen deficiency is associated with increased loss of teeth and bone. Doxycycline reduces collagenase activity/expression and enhances osteoblast activity, collagen production and bone formation. This two-year clinical trial assessed the efficacy of subantimicrobial dose doxycycline (SDD) in maintaining clinical periodontal heath in postmenopausal estrogen-deficient, osteopenic subjects with periodontitis.
Principal Findings:
Analyses of secondary outcomes of this clinical trial indicated that SDD may be of benefit in reducing progressive attachment loss in postmenopausal females; additional research is needed to confirm these findings.
Practical Implications:
SDD may be useful in postmenopausal osteopenic women with progressive attachment loss.
Introduction
Loss of bone mineral density in the skeleton (osteopenia/osteoporosis) has been associated with loss of periodontal support. Krall et al. (1994) showed a significant positive correlation between the number of teeth present and bone mineral density in the spine and radius. Von Wowern et al. (1994) reported that postmenopausal women with severe osteoporosis exhibited increased periodontal attachment loss compared to controls. Our group has demonstrated that non-smoking osteopenic/osteoporotic, estrogen-deficient periodontitis patients on periodontal maintenance had more bleeding on probing (43.8% versus 24.4%, p < 0.04), and a trend toward a higher frequency of ≥ 2.0 mm attachment loss over a 2-year period (3.8% versus 1.2%, p < 0.1) than estrogen-sufficient subjects (Reinhardt et al. 1999). Osteopenia or osteoporosis, after adjustment for age and menopausal status, is associated with an odds ratio of 2.0 (95% CI: 1.1−3.7) in relation to periodontitis in Japanese women (Inagaki et al. 2005).
Because osteoporosis usually requires bone-modulating pharmacotherapy, osteopenia (less severe bone loss than osteoporosis) is a more appropriate (i.e., an ethical) model in which to study the natural progression of periodontitis and host-modulating interventions. However, osteopenia has a weak association with clinical attachment loss in cross-sectional studies (Weyant et al. 1999, Tezal et al. 2000), even though one longitudinal study of bone mineral density in an osteopenic group showed a significant association with the number of progressive sites losing ≥ 3 mm of clinical attachment over a 3-year period (p = 0.001, Yoshihara et al. 2004).
Subantimicrobial dose doxycycline (SDD) as an adjunct to scaling/root planing (SRP) has been shown to be capable of improving clinical attachment levels (CAL), probing depths (PD) and lowering inflammation in general populations of patients with periodontitis (Caton et al. 2000, Caton et al. 2001, Novak et al. 2002, Lee et al. 2004, Preshaw et al. 2004, Gurkan et al. 2005). While reviews (Reddy et al. 2003) of research in this area conclude that adjunctive use of SDD with SRP is statistically more effective than SRP alone in reducing PD and increasing CAL, the clinical relevance of these improvements has been questioned in the opinion of some (Greenstein 2004). In clinical practice, automatic use of SDD with SRP for moderate-severe chronic periodontitis is not a universal scenario. However, it may be particularly appropriate to use SDD to help maintain longer-term periodontal attachment in vulnerable subjects (Oringer 2002). Postmenopausal osteopenic/osteoporotic estrogen-deficient women on periodontal maintenance therapy have been shown to be a vulnerable population (Reinhardt et al. 1999). Therefore, the goal of this paper was to determine the clinical efficacy of a two-year SDD regimen in postmenopausal, osteopenic, estrogen-deficient women on periodontal maintenance as part of a randomized, double-blind, placebo-controlled clinical trial designed to assess alveolar bone loss (primary outcome, companion paper).
Materials and Methods
Participants.
A detailed description of subject recruitment, screening, inclusion criteria, exclusion criteria, and demographic data has been included in a companion paper. Briefly, subjects were 45−70 years of age, osteopenic at the lumbar spine or femoral neck based on dual-energy x-ray absorptiometry (DEXA) scores with generalized moderate to advanced periodontitis and undergoing periodontal maintenance therapy. All subjects had at least 9 posterior teeth and at least two sites with ≥ 5 mm of probing depth and clinical attachment loss that bled on probing. Subjects could not have diseases that require regular drug therapy which would affect the inflammatory or immune responses, or bone remodeling.
Study Design.
This study was a two center (University of Nebraska Medical Center College of Dentistry and Stony Brook University School of Dental Medicine), double-blind, placebo-controlled, randomized 2-year clinical trial with each subject instructed to take all study medications daily for two years. Study participants, those administering the interventions and those assessing the outcomes were blinded to group assignment. The study had two treatment arms: 20 mg doxycycline twice daily (low-dose or subantimicrobial dose-doxycycline; SDD) and a placebo look-alike twice daily. Sixty-four eligible subjects were randomized into each treatment arm. All subjects received calcium and vitamin D supplements (1200 mg of calcium and 400 IU of vitamin D daily). All subjects received periodontal maintenance paid for by the study every 3−4 months throughout the clinical trial, delivered by the subjects' own dental care providers and not by the study clinicians.
Clinical Measurements.
Clinical measurements were taken at all posterior interproximal sites at baseline (before interventions), and every 6 months for 2 years. Supragingival plaque (PL) was recorded at each experimental site if present on the explorer tip after a single pass across the tooth surface. Relative clinical attachment level (RCAL) measurements were made with the Florida Disk Probe using 20 g of force (Florida Probe Corp., Gainesville, FL, USA). Baseline measurements were recorded using two passes. The Florida Probe software required that any site with a difference ≥ 1.0 mm between the two baseline passes be remeasured until the values for sequential validation passes were within 1.0 mm, and the deeper of the two measures within one mm was accepted. This tight validation was used to improve the accuracy of the baseline values from which all subsequent visits were compared. Measurements at the follow-up visits that varied from baseline by ≥ 2.0 mm were identified (Florida Probe software) and remeasured to confirm the change since this standard for attachment loss had been used in previous studies (Reinhardt et al. 1999, Caton et al. 2000, Preshaw et al. 2004). The final measurement was accepted where two sequential measurements were within 1.0 mm of each other when over the 2 mm threshold. The same examiner followed the same subjects throughout the longitudinal study (RAR at Nebraska and MSW at Stony Brook), since it has been reported that inter-examiner variation normally exceeds intra-examiner variation in probing depth, clinical attachment level and relative clinical attachment levels (Fleiss, 1986). Posterior interproximal sites which bled on probing (BOP) after the first pass were recorded. Finally, probing depths were recorded using a UNC-15 probe (Hu-Friedy, Chicago, IL, USA) with light probing force. Replicate measures for RCAL and PD were made at 6 and 18 month visits on 12 randomly-chosen subjects at each time point to estimate the standard deviation of replicate measures. Prior to beginning subject recruitment, the clinical examiners at Nebraska (RAR) and Stony Brook (MSW) were calibrated on a separate small group of subjects. A threshold of 85% of sites in agreement within ± 1 mm between examiners with respect to RCAL and probing depth measurement was met (88% and 100%, respectively) in this calibration exercise.
Statistical Analyses.
Statistical analyses were defined thoroughly in the companion paper. The primary endpoint of the clinical trial was alveolar bone density loss as measured by radiographic absorptiometry. The clinical endpoints reported in this paper were secondary endpoints. The power analysis was based on bone density parameters (primary endpoint), so the statistical power may have been less than ideal for clinical comparisons relative to other SDD clinical trials (Caton et al. 2000, Preshaw et al. 2004). The changes in RCAL and PD from baseline were each divided into 3 categories (improvement, no change, and disease progression) using thresholds defined by two standard deviations of replicate measures described in the previous section, rounded up to the nearest 0.5 mm for RCAL and 1.0 mm for PD. Changes beyond two times the standard deviation of replicate measurements are not expected to be due to measurement error or chance variation. Such changes over a 2-year period are of clinical concern, especially if disease progression is extrapolated over longer periods of periodontal maintenance. The thresholds defining significant changes at the site level for the Florida Probe RCAL change were ≥ 1.5 mm defining progression, ≤ −1.5 mm defining improvement (i.e., gain in RCAL) and values in between thresholds defining no change. A similar definition was used for UNC Probe PD measures using a threshold of 2 mm instead of 1.5 mm. A 2 mm threshold was chosen for PD because the two standard deviation threshold was slightly above one mm and PD was measured on a one mm measurement scale of the UNC-15 probe.
To account for the correlation among measures within a mouth over time, Generalized Estimating Equations (GEE) analysis (Liang & Zeger, 1986) was used to fit cumulative logistic regression models for the multinomial responses to compare the odds of more progressive disease (among the ordered categories of improvement, no change, and progression) over the treatment period between the SDD and placebo groups. Covariates in the regression models included time, treatment, and their interaction, where non-significant interaction terms were dropped from subsequent models. Stratification factors (baseline smoking status and study center) also were included in the regression models as was the baseline RCAL or PD measurement. Logistic regression models, with adjustment as described for RCAL and PD models, were used to compare the odds of PL and BOP between the treatment groups. The primary analysis was intent-to-treat (ITT). All available data were analyzed from all randomized subjects. Treatment effects also were investigated in a “per-protocol” analysis set that was defined using the same exclusion criteria as described in the companion paper. While the numbers of subjects remaining after per-protocol exclusions were considerably smaller than for the intent-to-treat groups (Table 1), both groups had similar characteristics including age, ethnicity, race, years postmenopausal, smoking, number of teeth and probing depths (data not shown). Reasons subjects were excluded for per-protocol analyses are summarized in Table 1. Both groups had similar numbers of subjects excluded. No subjects were deleted for active periodontal therapy in either group, although sites impacted by such treatment were deleted from the per-protocol set. No subject had more than 7 sites deleted for active periodontal therapy during any 6-month period. Exploratory subgroup analyses were performed as defined by baseline smoking status, time since onset of menopause (≤ 5 years or > 5 years), study center, patient adherence to study medication, individual “per-protocol” criteria, maxilla versus mandible, site location, and baseline probing depth and alveolar bone height measures. Tests of interactions were used to identify significant subgroup effects, and only those subgroup comparisons with a significant test of interaction and a sustained affect on SDD performance over time, and that could be justified biologically, are reported. No formal adjustment to the alpha level was made for the multiple tests performed.
Table 1.
Reasons for Excluding Subjects from Per-Protocol Analyses at Each Study Visit
| Exclusion Factor | Placebo Visit | SDD Visit | ||||||
|---|---|---|---|---|---|---|---|---|
| 6-mo | 12-mo | 18-mo | 24-mo | 6-mo | 12-mo | 18-mo | 24-mo | |
| Patient withdrawal before designated visit | n=2 | 2 | 2 | 2 | 7 | 9 | 13 | 13 |
| Patient missing probing data due to antibiotic premedication and adherent to study drug, Ca/Vit D and no significant concomitant med use | n=0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Patient not adherent to study drug, Ca/Vit D and no significant concomitant med use | n=15 | 21 | 20 | 20 | 9 | 11 | 10 | 10 |
| Patient not adherent to study drug, Ca/Vit D and significant concomitant med use | n=1 | 3 | 6 | 9 | 2 | 2 | 3 | 4 |
| Patient adherent to study drug, Ca/Vit D and significant concomitant med use | n=8 | 10 | 12 | 14 | 10 | 10 | 11 | 13 |
Results
Fifty-one of 64 SDD subjects (80%) and 62 of 64 placebo subjects (97%) finished the study (see flow diagram in companion paper). As for concomitant medications taken during the study which could affect bone metabolism, ≤ 2% of subjects took hormone replacement therapy, ≤ 8% took bisphosphonates or steroids, ≤ 5% took more than 2 courses of antibiotics, ≤ 2% took daily nonsteroidal anti-inflammatory drugs (NSAIDs), ≤ 6% took NSAIDs or high-dose (> 325 mg) aspirin for 30 days or more, and ≤ 22% took thyroid medications in each treatment group in each 6-month study period. This resulted in a total of 23 placebo patients and 17 SDD patients who took significant concomitant medications during the study period. Observations from these subjects were excluded in the “per-protocol” analyses.
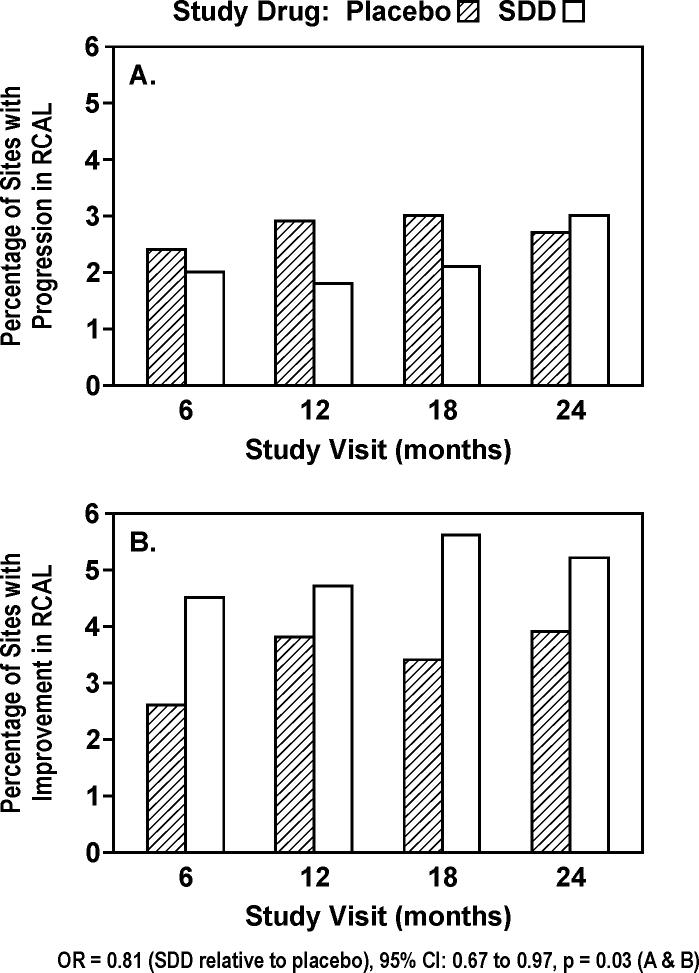
The vast majority of sites (93−95% in placebo and 92−94% in SDD as means over 4 post-baseline visits) did not manifest significant RCAL change across the 2-year clinical trial. Based on regression modeling, the odds of more disease progression (continuum from improvement [Figure 1B] to no change to progression [Figure 1A]) were 19% lower (2.1% absolute difference in number of sites) for subjects receiving SDD relative to placebo (OR = 0.81, 95% confidence interval: 0.67 to 0.97, p = 0.03). A 20% (relative) reduction in the percentage of sites showing significant RCAL progression and a 46% (relative) increase in the percentage of sites showing significant RCAL improvement were seen in the SDD group compared to the placebo group over 2 years. There was evidence that the treatment effect over time differed by study center (p = 0.05). SDD demonstrated a significant reduction in the odds of more progressive disease among University of Nebraska Medical Center (UMMC) College of Dentistry subjects across the 24-month treatment period (OR = 0.77, 95% CI: 0.61 to 0.99, p = 0.04). At the School of Dental Medicine at Stony Brook University, the treatment effect differed significantly over time (p = 0.02), where SDD was associated with a 28% reduction in the odds of more progressive disease over the first 18 months of follow-up (OR = 0.72, 95% CI: 0.54 to 0.96, p = 0.03), which was not sustained through the 24-month visit (OR = 1.32, 95% CI: 0.87 to 2.02, p = 0.2). Among the subjects who were adherent to the protocol (“per-protocol” analysis), the odds of more progressive disease were 21% lower for subjects receiving SDD relative to placebo (OR = 0.79, 95% CI: 0.62 to 1.01, p = 0.07).
Figure 1.
Percent of sites in study subjects (placebo or subantimicrobial dose doxycycline [SDD]) which demonstrated significant change (≥ 1.5 mm loss [A] or gain [B]) in relative clinical attachment level (RCAL) during 2 years of periodontal maintenance (based on intent-to-treat). 92−95% of sites showed no significant change at each of the study visits. Significant change was based on two times the standard deviation of replicate measurements.
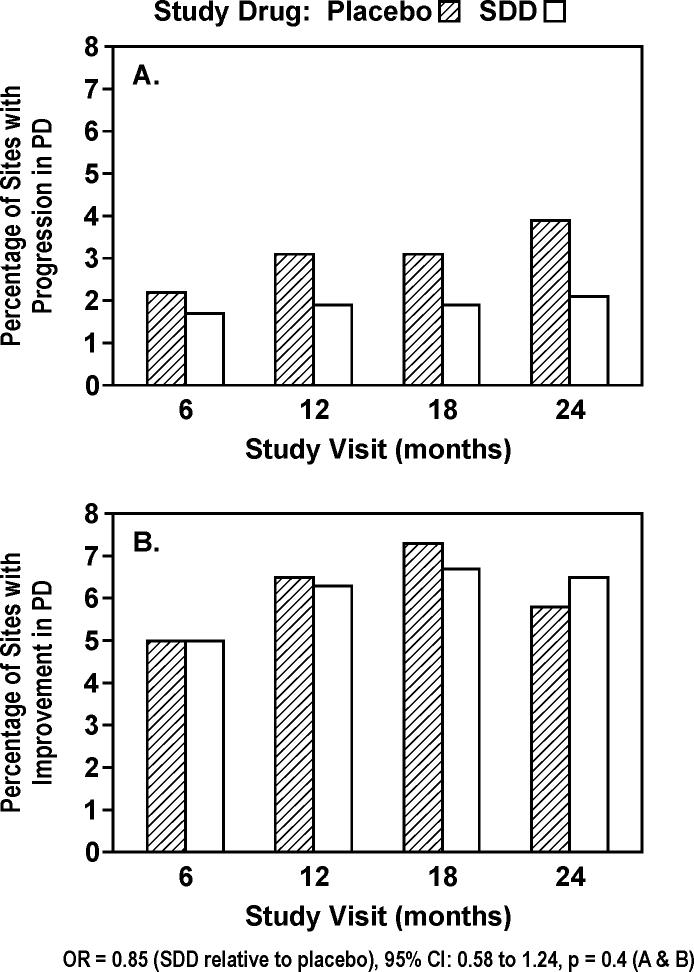
Most sites showed no change in PD across time (90−93%). The odds of more progressive PD change were 15% lower in the SDD group relative to the placebo group, but this was not statistically significant (OR = 0.85, 95% CI: 0.58 to 1.24, p = 0.4; Figure 2). The treatment effect differed over time depending on significant concomitant medication use (p = 0.03, time by treatment by concomitant medication interaction). Among subjects not taking significant concomitant medications (exploratory subgroup analysis), the odds of more progressive disease at 24-months were decreased 43% in SDD versus placebo subjects (OR = 0.57, 95% CI: 0.34 to 0.97, p = 0.04), while the effect at other time points among non-users and over the entire treatment period among users was not significant. There was no significant effect of SDD relative to placebo among the “per-protocol” analysis set (OR = 0.82, 95% CI: 0.50 to 1.32, p = 0.4).
Figure 2.
Percent of sites in study subjects which demonstrated significant change (≥ 2.0 mm loss [A] or gain [B]) in probing depth (PD) during 2 years of periodontal maintenance (intent-to-treat). 90−93% of sites showed no significant change at each of the study visits. Significant change was based on two times the standard deviation of replicate measurements.
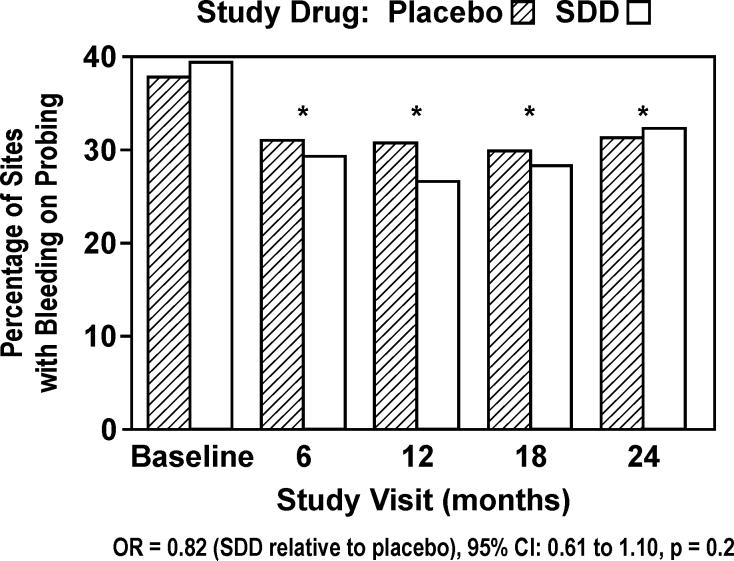
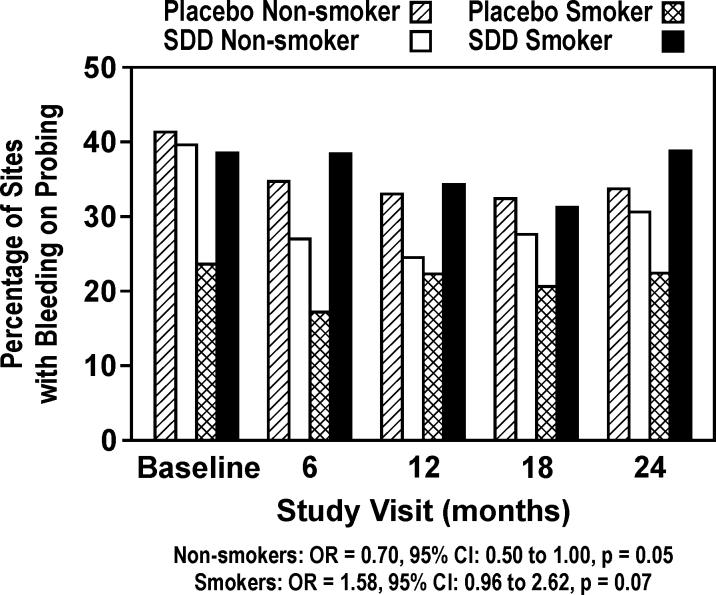
The odds of BOP were 18% lower for subjects receiving SDD relative to placebo, which was not statistically significant (OR = 0.82, 95% CI: 0.61 to 1.10, p = 0.2; Figure 3). However, based on logistic regression modeling after adjustment for the treatment effect, BOP was significantly reduced in both groups (p < 0.0001) following the baseline visit (Figure 3). Exploratory subgroup analyses showed that the effect of study drug differed by smoking status (p = 0.01), where SDD was associated with a reduction in the odds of BOP relative to placebo for non-smokers (OR = 0.70, 95% CI: 0.50 to 1.00, p = 0.05), and a marginal increase for smokers (OR = 1.58, 95% CI: 0.96 to 2.62, p = 0.07; Figure 4). The effect of SDD over time differed by site location (p = 0.01), where the greatest reductions in the odds of BOP with SDD were seen between the molars after 6, 12 and 18 months (OR ranging from 0.66 to 0.74, p ≤ 0.1 for each), while no significant effect was observed at 24 months. Among the subjects who were adherent to the protocol (“per-protocol” analysis), the odds of BOP were 34% lower for subjects receiving SDD relative to placebo (OR = 0.66, 95% CI: 0.44 to 1.00, p = 0.05).
Figure 3.
Percent of sites in study subjects which bled on probing during 2 years of periodontal maintenance (intent-to-treat). * indicates significant reduction from baseline across both treatment groups with p < 0.0001.
Figure 4.
Percent of sites in study subjects according to smoking status which bled on probing during 2 years of periodontal maintenance (exploratory subgroup analyses; placebo non-smoker n=49, SDD non-smoker n=44, placebo smoker n=13, SDD smoker n=11). A significant difference between nonsmoker groups existed at p = 0.05 across all time points combined.
When sites with RCAL changes were further analyzed according to PD and BOP characteristics, placebo sites that demonstrated an improvement in RCAL (n=388) showed a mean PD change of −0.33 mm (improvement) compared to −0.13 mm and +0.019 mm (increase in PD) among sites that demonstrated no change in RCAL (n=10,680) or an increase in RCAL (n=312), respectively. The percentage of sites with BOP was 22%, 31% and 33% across RCAL change categories of decrease, no change, and increase, respectively. Among the SDD sites that demonstrated an improvement in RCAL (n=517), the mean PD change was −0.46 mm compared to −0.17 mm and +0.10 mm among sites that demonstrated no change in RCAL (n=9,641) or an increase in RCAL (n=229), respectively. The percentage of sites with BOP was 22%, 29%, and 41% across RCAL change categories of decrease, no change, and increase, respectively. Therefore, it appears that improvement in RCAL was associated with reductions on average in PD and lower percentages of BOP, although fewer than 15% of sites that showed improvements, beyond the specified thresholds, in RCAL also showed improvements, beyond the specified thresholds, in PD. It is also important to note that RCAL and PD were measured with different instruments, so assessment of the agreement between changes in clinical attachment and probing depth was not made in this study.
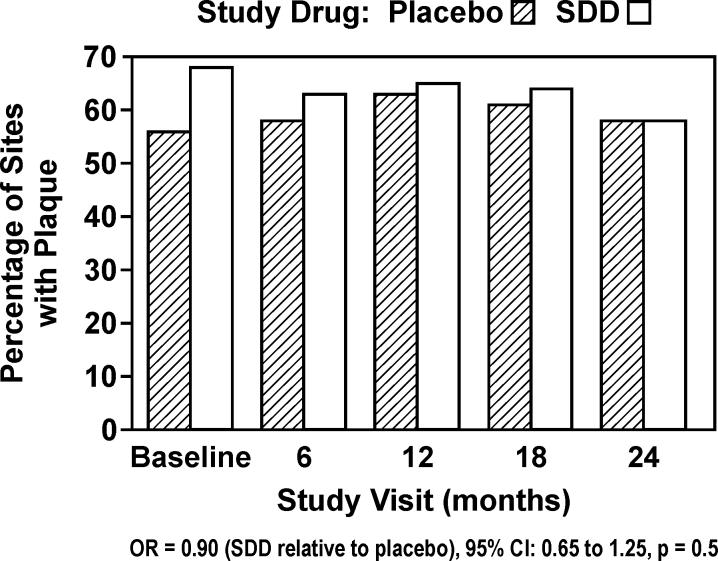
Supragingival plaque levels were high in this group of subjects (56−68% across time points and study drug groups). There was no significant effect for PL in subjects receiving SDD relative to placebo (OR = 0.90, 95% CI: 0.65 to 1.25, p = 0.5; Figure 5). There was no significant effect of SDD relative to placebo among the “per-protocol” analysis set (OR = 0.86, 95% CI: 0.56 to 1.34, p = 0.5).
Figure 5.
Percent of sites in study subjects which demonstrated supragingival interproximal plaque (PL) during 2 years of periodontal maintenance (intent-to-treat).
Discussion
Subjects in this study represented a mostly stable (non-progressive) group on periodontal maintenance, in spite of high plaque (range of means over all visits for entire study population: 56−68%) and bleeding on probing (27−39%). Only 4 teeth were lost due to periodontal disease in the SDD group and 7 teeth in the placebo group. Clinical attachment levels in 92 to 95% of sites did not change in a significant way (≥ 1.5 mm) over the 2-year period. Periodontal maintenance at no cost to the patient may account, in part, for the high compliance with the 3−4 month regimen over the 2-year study, resulting in very stable attachment levels in this vulnerable population. An average of 2.5% of posterior interproximal sites in non-smoking placebo subjects and 2.1% of sites in non-smoking SDD subjects lost ≥ 1.5 mm clinical attachment across 2 years. This compares to 3.8% of similar sites in non-smoking periodontitis, osteopenic/osteoporotic, estrogen-deficient patients losing ≥ 2.0 mm of clinical attachment over 2 years on maintenance (Reinhardt et al. 1999) and 1.2% of sites/year in non-smoking periodontitis patients (Kaldahl et al. 1996) on similar maintenance protocols. Considering the lower threshold for clinical progression (≥ 1.5 mm, based on two times the standard deviation of replicate measures [0.69 mm]) in the current study population, the periodontal stability in this potentially vulnerable population is impressive. Across all time points and subjects (smokers and non-smokers) combined, 3.4% of placebo sites demonstrated improvements and 2.7% demonstrated progression in RCAL compared to 5.0% and 2.2% of SDD sites, respectively. Therefore, 2.1% more sites (absolute difference) among SDD patients demonstrated improvements or a lack of progression compared to placebo patients, which represents a large portion of sites normally showing disease progression in this group of patients (Reinhardt et al. 1999). Furthermore, among the 64 SDD patients, 17 (27%) had 4 or more sites that showed improvement and 1 or fewer sites that showed progression compared to 6 (9%) of the 64 placebo patients. A potential error in RCAL measurement which could have been introduced by the 1.5 mm threshold, after remeasuring sites during the study only when they differed from baseline by ≥ 2 mm, is that a small number of sites measuring a > 1.5 to < 2.0 mm change from baseline would not have been remeasured. However, this error would have been made in both SDD and placebo subjects. Some increased progression of attachment loss may be suggested at 24 months (Figure 1A), which may be due to drug regimen fatigue noted in earlier flurbiprofen studies (Williams et al. 1989).
Including smokers (comprising 20.3% of subjects in drug and placebo groups) in the analyses did not affect SDD's ability to promote periodontal stability (cumulative logistic model, p = 0.5, smoking status by treatment interaction), in spite of the fact that smoking has been shown to cause more attachment loss during periodontal maintenance (Ah et al. 1994), including postmenopausal females (Payne et al. 2000). The general efficacy of SDD in maintaining RCAL stability was supported by the finding that none of the other exploratory subgroup analyses of systemic (time since menopause, concomitant medication use, aspirin use), anatomic (maxilla/mandible, tooth location, baseline probing depth/bone height) or behavioral considerations (compliance with protocol or study drug use) significantly impacted SDD positive effects, although the power to detect such modifications is limited.
In contrast to SDD's significant effect on RCAL stability, the effect of SDD on probing depth was not significant (Figure 2). The only subgroup that showed a significant SDD effect was subjects not taking significant concomitant medication (43% reduction in SDD versus placebo at 24 months). This moderate-severe periodontitis population was on periodontal maintenance following active therapy, so mean posterior interproximal probing depths in both groups at baseline were relatively shallow (3.83 +/- 1.19 mm in placebo group and 3.83 +/- 1.14 mm in SDD group). In addition, probing depth appears to be less appropriate for tracking periodontal stability during periodontal maintenance than clinical attachment levels (Kaldahl et al. 1996). Both SDD and placebo were associated with a significant reduction in BOP after baseline (Figure 3). This may be partially due to the research protocol which included 3-month periodontal maintenance at no cost to the patient, plus additional 6-month periodontal examinations where plaque control and BOP were measured and discussed. It is quite conceivable that periodontal health was more in focus for these patients during the 2-year study period. However, supragingival plaque levels remained high during all post-baseline examinations (Figure 5), suggesting that factors other than home oral hygiene may explain BOP reductions. Heightened compliance with periodontal maintenance visits and associated subgingival debridement probably played a role in both BOP reductions and RCAL stability.
SDD affected BOP differently in smokers versus non-smokers (Figure 4, exploratory subgroup analyses), where SDD reduced BOP in non-smokers relative to placebo but marginally increased it in smokers. This effect in smokers may reflect the fact that across all time points including baseline, the smokers receiving placebo had a much lower probability of BOP compared to SDD subjects (17−22% among placebo smokers versus 31−39% among SDD smokers). The model was adjusted for baseline BOP, but some residual confounding by baseline inflammatory status may have existed, not captured by baseline BOP adjustments. In addition, while smokers generally exhibit greater periodontitis progression than non-smokers (Machtei et al. 1999), there appears to be a disconnect with BOP which is strongly suppressed in smokers (Dietrich et al. 2004). BOP reduction in SDD patients has been reported previously (Ashley 1999, Mohammad et al. 2005). In addition, tetracyclines have been shown to reduce proinflammatory molecules like interleukin (IL)-1 (Solomon et al. 2000), IL-6 (Kirkwood et al. 2003), inducible nitric oxide synthase (Sadowski & Steinmeyer 2001), and MMP activity and expression (Golub et al. 1998). Therefore, SDD reduction of clinical inflammation as modeled by BOP was expected. Reductions in BOP were greatest where BOP is traditionally highest, in the most posterior interproximal sites (44.5% BOP for baseline molar-molar was reduced by 22, 29 and 27% at 6-, 12- and 18-month visits, respectively). The p values used in the above analyses were not adjusted for multiple comparisons. An ad-hoc adjustment, such as using an alpha level of 0.01 instead of 0.05, does not necessarily guarantee an experiment-wise nominal type I error rate. Statistical adjustments for the analysis of multiple endpoints are usually of limited value except as informal guides for interpretation (Pocock 1997). Subgroup analyses are exploratory and are presented to generate hypotheses regarding differential effects (of SDD in subgroups of patients). The subgroup analysis results are not confirmatory and given the multiple hypothesis tests performed, should be interpreted cautiously.
Taken together, data from this study indicate that SDD use in osteopenic, postmenopausal, estrogen-deficient females may be beneficial in maintaining attachment levels and reducing progressive disease during periodontal maintenance in this vulnerable population. ITT was the primary analysis which showed a significant reduction in progressive attachment loss in the SDD group (OR = 0.81, 95% CI: 0.67−0.97, p = 0.03; 2.1% more sites with improvements or a lack of progression as calculated in the first paragraph of this Discussion), in spite of numerous factors which “raised the bar” against showing an effect; i.e., inclusion of all subjects who: a) dropped out before the study was completed; b) did not comply with taking their medication; c) used confounding medications (e.g., bisphosphonates, estrogen or NSAIDs); and d) received active periodontal therapy. In addition all subjects received optimal supplementation with calcium and vitamin D and were on tight periodontal maintenance. Therefore, the RCAL findings support the effectiveness of this drug in this maintenance population and argues that SDD (especially with recent one/day dosing formulations) be considered for postmenopausal women on maintenance where clinical attachment levels are not well controlled. The drug also appears to reduce periodontal inflammation independent of plaque levels in non-smokers and patients who are adherent with the study protocol, although secondary endpoint and subgroup analysis results must be interpreted cautiously given the multiple hypothesis tests that were performed and multiple subgroups that were investigated.
Acknowledgements:
We acknowledge and thank the following individuals for their hard work and dedication to this clinical trial: Alison Lahners for her role as project assistant and registrar, Julie Layton as clinical research assistant, Eugene Boilsen as database administrator, Mary Morris as project manager, and Marian Schmid for her role as research technologist. We thank the Nebraska Periodontitis Referral Network (PRN) for referring subjects for this clinical trial. We also acknowledge Susan McCoy for manuscript preparation and Kim Theesen for preparation of graphics. SDD and placebo tablets were provided by CollaGenex Pharmaceuticals, Inc. (Newton, PA).
Source of Funding: The project described was supported by Grant Number R01DE012872 (Dr. Jeffrey Payne, PI) from the National Institute of Dental & Craniofacial Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental & Craniofacial Research or the National Institutes of Health.
Conflict of Interest: Lorne M. Golub is listed as an inventor on several patents for the drug mentioned in this article and these patents have been fully assigned to his institution, State University of New York at Stony Brook. Lorne M. Golub is a consultant to CollaGenex Pharmaceuticals, Inc. and the Fund for Autoimmune Diseases Research.
Maria E. Ryan is also a consultant to CollaGenex Pharmaceuticals.
References
- Ah MK, Johnson GK, Kaldahl WB, Patil KD, Kalkwarf KL. The effect of smoking on the response to periodontal therapy. Journal of Clinical Periodontology. 1994;21:91–97. doi: 10.1111/j.1600-051x.1994.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Ashley RA. Clinical trials of a matrix metalloproteinase inhibitor in human periodontal disease. SDD Clinical Research Team. Annals of the New York Academy of Sciences. 1999;30:335–346. doi: 10.1111/j.1749-6632.1999.tb07693.x. [DOI] [PubMed] [Google Scholar]
- Caton JG, Ciancio SG, Blieden TM, Bradshaw M, Crout RJ, Hefti AF, Massaro JM, Polson AM, Thomas J, Walker C. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. Journal of Periodontology. 2000;71:521–532. doi: 10.1902/jop.2000.71.4.521. [DOI] [PubMed] [Google Scholar]
- Caton JG, Ciancio SG, Blieden TM, Bradshaw M, Crout RJ, Hefti AF, Massaro JM, Polson AM, Thomas J, Walker C. Subantimicrobial dose doxycycline as an adjunct to scaling and root planing: post-treatment effects. Journal of Clinical Periodontology. 2001;28:782–789. doi: 10.1034/j.1600-051x.2001.280810.x. [DOI] [PubMed] [Google Scholar]
- Dietrich T, Bernimoulin JP, Glynn RJ. The effect of cigarette smoking on gingival bleeding. Journal of Periodontology. 2004;75:16–22. doi: 10.1902/jop.2004.75.1.16. [DOI] [PubMed] [Google Scholar]
- Fleiss JL. Reliability of measurement. In: Fleiss JL, editor. The Design and Analysis of Clinical Experiments. John Wiley and Sons; New York: 1986. pp. 1–32. [Google Scholar]
- Golub LM, Lee H-M, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Advances in Dental Research. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- Greenstein G. Efficacy of subantimicrobial-dose doxycycline in the treatment of periodontal diseases: a critical evaluation. International Journal of Periodontics and Restorative Dentistry. 2004;24:528–543. [PubMed] [Google Scholar]
- Gurkan A, Cinarcik S, Huseyinov A. Adjunctive subantimicrobial dose doxycycline: effect on clinical parameters and gingival crevicular fluid transforming growth factor-beta levels in severe generalized chronic periodontitis. Journal of Clinical Periodontology. 2005;32:244–253. doi: 10.1111/j.1600-051X.2005.00663.x. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Kurosu Y, Yoshinari N, Noguchi T, Krall EA, Garcia RI. Efficacy of periodontal disease and tooth loss to screen for low bone mineral density in Japanese women. Calcified Tissue International. 2005;77:9–14. doi: 10.1007/s00223-004-0275-x. [DOI] [PubMed] [Google Scholar]
- Kaldahl WB, Kalkwarf KL, Patil KD, Molvar M, Dyer JK. Long-term evaluation of periodontal therapy: II. Incidence of sites breaking down. Journal of Periodontology. 1996;67:103–108. doi: 10.1902/jop.1996.67.2.103. [DOI] [PubMed] [Google Scholar]
- Kirkwood K, Martin T, Andreadis ST, Kim YJ. Chemically modified tetracyclines selectively inhibit IL-6 expression in osteoblasts by decreasing mRNA stability. Biochemical Pharmacology. 2003;66:1809–1819. doi: 10.1016/s0006-2952(03)00450-7. [DOI] [PubMed] [Google Scholar]
- Krall EA, Dawson-Hughes B, Papas A, Garcia RI. Tooth loss and skeletal bone density in healthy postmenopausal women. Osteoporosis International. 1994;4:104–109. doi: 10.1007/BF01623233. [DOI] [PubMed] [Google Scholar]
- Lee JY, Lee YM, Shin SY, Seol YJ, Ku Y, Rhyu IC, Chung CP, Han SB. Effect of subantimicrobial dose doxycycline as an effective adjunct to scaling and root planing. Journal of Periodontology. 2004;75:1500–1508. doi: 10.1902/jop.2004.75.11.1500. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Machtei EE, Hausmann E, Dunford R, Grossi S, Ho A, Davis G, Chandler J, Zambon J, Genco RJ. Longitudinal study of predictive factors for periodontal disease and tooth loss. Journal of Clinical Periodontology. 1999;26:374–380. doi: 10.1034/j.1600-051x.1999.260607.x. [DOI] [PubMed] [Google Scholar]
- Mohammad AR, Preshaw PM, Bradshaw MH, Hefti AF, Powala CV, Romanowicz M. Adjunctive subantimicrobial dose doxycycline in the management of institutionalized geriatric patients with chronic periodontitis. Gerodontology. 2005;22:37–43. doi: 10.1111/j.1741-2358.2004.00044.x. [DOI] [PubMed] [Google Scholar]
- Novak MJ, Johns LP, Miller RC, Bradshaw MH. Adjunctive benefits of subantimicrobial dose doxycycline in the management of severe generalized, chronic periodontitis. Journal of Periodontology. 2002;73:762–769. doi: 10.1902/jop.2002.73.7.762. [DOI] [PubMed] [Google Scholar]
- Oringer RJ. Modulation of the host response in periodontal therapy. Journal of Periodontology. 2002;73:460–470. doi: 10.1902/jop.2002.73.4.460. [DOI] [PubMed] [Google Scholar]
- Payne JB, Reinhardt RA, Nummikoski PV, Dunning DG, Patil KD. The association of cigarette smoking with alveolar bone loss in postmenopausal females. Journal of Clinical Periodontology. 2000;27:658–664. doi: 10.1034/j.1600-051x.2000.027009658.x. [DOI] [PubMed] [Google Scholar]
- Pocock SJ. Clinical trials with multiple outcomes: a statistical perspective on their design, analysis, and interpretation. Controlled Clinical Trials. 1997;18:530–545. doi: 10.1016/s0197-2456(97)00008-1. [DOI] [PubMed] [Google Scholar]
- Preshaw PM, Hefti AF, Novak MJ, Michalowicz BS, Pihlstrom BL, Schoor R, Trummel CL, Dean J, Van Dyke TE, Walker CB, Bradshaw MH. Subantimicrobial dose doxycycline enhances the efficacy of scaling and root planing in chronic periodontitis: a multicenter trial. Journal of Periodontology. 2004;75:1068–1076. doi: 10.1902/jop.2004.75.8.1068. [DOI] [PubMed] [Google Scholar]
- Reddy MS, Geurs NC, Gunsolley JC. Periodontal host modulation with antiproteinase, anti-inflammatory and bone-sparing agents. A systematic review. Annals of Periodontology. 2003;8:12–37. doi: 10.1902/annals.2003.8.1.12. [DOI] [PubMed] [Google Scholar]
- Reinhardt RA, Payne JB, Maze CA, Patil KD, Gallagher SJ, Mattson JS. Influence of estrogen and osteopenia/osteoporosis on clinical periodontitis in postmenopausal women. Journal of Periodontology. 1999;70:823–828. doi: 10.1902/jop.1999.70.8.823. [DOI] [PubMed] [Google Scholar]
- Sadowski T, Steinmeyer J. Minocycline inhibits the production of inducible nitric oxide synthase in articular chondrocytes. Journal of Rheumatology. 2001;28:336–340. [PubMed] [Google Scholar]
- Solomon A, Rosenblatt M, Li DQ, Liu Z, Monroy D, Ji Z, Lokeshwar BL, Pflugfelder SC. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Investigative Ophthalmology and Visual Science. 2000;41:2544–2557. [PubMed] [Google Scholar]
- Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. Journal of Periodontology. 2000;71:1492–1498. doi: 10.1902/jop.2000.71.9.1492. [DOI] [PubMed] [Google Scholar]
- von Wowern N, Klausen B, Kollerup G. Osteoporosis: a risk factor in periodontal disease. Journal of Periodontology. 1994;65:1134–1138. doi: 10.1902/jop.1994.65.12.1134. [DOI] [PubMed] [Google Scholar]
- Weyant RJ, Pearlstein ME, Churak AP, Forrest K, Famili P, Cauley JA. The association between osteopenia and periodontal attachment loss in older women. Journal of Periodontology. 1999;73:298–301. doi: 10.1902/jop.1999.70.9.982. [DOI] [PubMed] [Google Scholar]
- Williams RC, Jeffcoat MK, Howell TH, Rolla A, Stubbs D, Teoh KW, Reddy MS, Goldhaber P. Altering the progression of human alveolar bone loss with the non-steroidal anti-inflammatory drug flurbiprofen. Journal of Periodontology. 1989;60:485–490. doi: 10.1902/jop.1989.60.9.485. [DOI] [PubMed] [Google Scholar]
- Yoshihara A, Seida Y, Hanada N, Miyazaki H. A longitudinal study of the relationship between periodontal disease and bone mineral density in community-dwelling older adults. Journal of Clinical Periodontology. 2004;31:680–684. doi: 10.1111/j.1600-051X.2004.00548.x. [DOI] [PubMed] [Google Scholar]