Abstract
Background
Metabolic syndrome, insulin resistance and diabetes are associated with an increased risk of cardiovascular disease. Niacin is known to increase insulin resistance, and have adverse effects on blood glucose levels, but to have beneficial effects on plasma lipids and lipoproteins. We therefore aimed to determine whether intensive lipid therapy with a niacin-containing regimen would have a beneficial effect on cardiovascular disease, despite an expected increase in plasma glucose and insulin resistance in subjects with the metabolic syndrome, insulin resistance or abnormal fasting plasma glucose levels.
Methods
The effect of three years’ treatment with niacin plus simvastatin (N+S) on both angiographic and clinical outcomes were analyzed in the 160 subjects with coronary artery disease (CAD) and low levels of high density lipoproteins (HDL) from the HDL-Atherosclerosis Treatment Study (HATS). A subgroup analysis was performed on the basis of: (1) the presence or absence of the metabolic syndrome, (2) higher or lower insulin resistance, and (3) the presence or absence of impaired fasting glucose or diabetes (dysglycemia). Individuals classified as having the MS, increased insulin resistance or dysglycemia would be expected to have increased cardiovascular risk.
Results
N+S reduced the change in mean proximal percent stenosis (Δ%S) compared to placebo (PL) in subjects with the metabolic syndrome (Δ%Sprox 0.3 vs 3.0, p=0.003) and in the more insulin resistant group of subjects (Δ%Sprox 0.5 vs 2.7, p=0.001), while subjects with dysglycemia (impaired fasting glucose or diabetes) showed a lesser benefit (Δ%Sprox 1 vs 3.2, p=0.13). These changes occurred despite increased in-treatment fasting glucose levels (3%), fasting insulin (19%) and decreased insulin sensitivity (−10%). Overall primary clinical events were reduced by 60% with N+S compared to PL (p=0.02). A similar reduction of the rate of primary events was seen in patients with metabolic syndrome, insulin resistance, and to a lesser extent in patients with dysglycemia in the N+S group compared to PL.
Conclusions
These data indicate that, in CAD patients with low HDL, treating the atherogenic dyslipidemia with a combination of N+S leads to substantial benefits in terms of stenosis progression and clinical events, independently of whether the patient has the metabolic syndrome or is insulin resistant. Over a 3 year period, the beneficial effect of niacin in combination with simvastatin appears to offset the modest adverse effect of niacin on glucose metabolism and insulin resistance in at higher risk patients, as long as careful attention is paid to glycemic control.
Keywords: Metabolic syndrome, dyslipidemia, lipid therapy, cardiovascular disease, insulin resistance
Introduction
The term metabolic syndrome (MS) defines a clustering of cardiovascular risk factors, which includes dyslipidemia, hypertension, visceral adiposity, glucose intolerance/insulin resistance, hypercoagulation, and inflammation (1). From large population studies it appears that the presence of MS increases cardiovascular risk by 2–4 fold (2,3). Individuals with the MS have been shown to be at increased risk of coronary heart disease (3), cardiovascular disease and mortality (4), and to benefit more from treatment, in terms of cardiovascular events and mortality, compared to subjects without the MS. A post-hoc subgroup analysis of the Scandinavian Simvastatin Survival Study (4S) (5) evaluated the relative impact of the “atherogenic lipid triad” (high levels of LDL cholesterol, low levels of HDL cholesterol, and high levels of triglycerides (6)) on clinical outcome. Patients characterized by the lipid triad had greater proportions of other features of MS, such as increased BMI, hypertension, and diabetes. Events were comparably reduced by simvastatin treatment relative to placebo, in patients with and without MS. However, because patients with MS were at higher absolute risk, the absolute benefit from simvastatin treatment was greater. Similar results, indicating that treating groups enriched with subjects with the MS leads more benefit in terms of outcome, came from various studies on statins (7) as well as on fibrates (8–10).
Whether the MS is mediated by a common underlying pathophysiology is still debated. Although potential contributors such as central obesity and inflammatory mediators have been suggested as driving the abnormalities related to MS (11, 12), insulin resistance also has been proposed as the unifying etiologic factor (13). If so, reducing insulin resistance should be a major target for therapy, and a worsening of insulin resistance would be expected to have adverse consequences.
Niacin reduces plasma triglycerides, increases HDL cholesterol and reduces LDL cholesterol modestly (14). Thus, it would seem to be an ideal drug for treating the dyslipidemia that is associated with the MS (15), particularly when used together with a statin, which would reduce LDL cholesterol levels further. However, niacin increases insulin resistance and can increase blood glucose levels (16). Previous reports from the Assessment of Diabetes Control and Evaluation of the Efficacy Niaspan Trial (ADVENT (17)), the Arterial Disease Multiple Intervention Study (ADMIT) (18) and from HDL-Atherosclerosis Treatment Study (HATS) (19), have shown that the modest increase in glucose level due to niacin treatment could be easily counteracted by adjusting the diet, exercise and dose of antidiabetic medication. However, these strategies are unlikely to benefit insulin resistance.
We therefore sought to determine whether aggressive treatment of dyslipidemia with niacin and a statin might be beneficial to high-risk subgroups of patients with increased insulin resistance, despite an expected worsening in glucose metabolism and insulin sensitivity. To this end, the HATS database (20) was analyzed to determine the impact of aggressive lipid lowering on both angiographic and clinical endpoints in subgroups of subjects with increased cardiovascular risk. Subjects were then separately classified according to three dichotomous, non- mutually exclusive metabolic variables: 1) presence or absence of MS, 2) higher or lower insulin resistance, and 3) normal or impaired fasting glucose.
Methods
Trial design
The design (21) and the results (20) of the HATS study have been described elsewhere. Briefly, HATS was a double-blind placebo controlled trial conducted on 160 patients with coronary artery disease, low HDL cholesterol and normal LDL-cholesterol, aimed at assessing the value of lipid altering and/or antioxidant therapy in patients with coronary artery disease and low HDL. Subjects were randomized in a factorial design to receive niacin plus simvastatin (N+S) or their placebo and antioxidant vitamins or their placebo. The end points were angiographic (measured change in mean proximal coronary stenosis) and clinical (time to first incidence of death from coronary causes, myocardial infarction, stroke or revascularization for progressive ischemia). A regression (−0.4%S) of the average stenosis with the N+S regimen was seen, while a progression of the stenosis was observed in the N+S plus antioxidants (0.7%S), in the antioxidants (1.8%S) and in the placebo (3.9%S) groups. The risk of the composite clinical endpoint was 60% lower in the N+S factorial group than in the placebo group; the antioxidant factorial treatment group showed no risk reduction when compared to placebo. The authors concluded that the therapy with N+S provides definite clinical and angiographic benefits, while there were no significant benefits from antioxidant therapy.
Because the antioxidant limb of the study was negative, subjects were divided in two groups for the purpose of the present analysis: 1) a group receiving N+S with either placebo or active antioxidant agents (n=80), and 2) a group receiving placebo for niacin and simvastatin with either active antioxidants or placebo (PL, n=80). Subjects were then classified according to their metabolic syndrome status (yes/no), insulin resistance status (more or less insulin resistant), and fasting glycemia status (normoglycemia or impaired fasting glycemia/diabetes). Therefore there was considerable overlap of subjects in these three categories.
Definition of Metabolic Syndrome
According to the National Cholesterol Education Program (NCEP) Expert Panel on the Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III (ATPIII)) (22), the MS definition was met when three or more of the following five criteria were satisfied: 1) HDL cholesterol <40 mg/dl in men and <50 mg/dl in women; 2) triglycerides ≥ 150 mg/dl; 3) hypertension, current or previously diagnosed, or blood pressure ≥ 130/85 mmHg; 4) fasting glucose ≥ 100 mg/dl, or previously-diagnosed diabetes now under treatment; 5) waist circumference >102 cm in men and >88 cm in women.
Waist circumference
Waist circumference was not measured in HATS, so it was derived from the BMI value, using a linear regression equation of BMI and waist circumference in all 2503 subjects in the VA-HIT study (23): waist (cm) = 2.096 * BMI (Kg/m2) + 40.355 (r = 0.839)*. (*Personal communication, Robins SJ, Bloomfield H.). A potential shortcoming is that these subjects were not aged and sex-matched with those in HATS.
Insulin sensitivity
Insulin sensitivity was calculated using the updated homeostatic model assessment (HOMA2), available on the website of the Diabetes Trial Unit of University of Oxford (www.dtu.ox.ac.uk) (24). The insulin sensitivity index provided by the model is expressed as a percentage of a young normal reference population (100%).
Statistical analysis
The 2×2 factorial randomization to lipid and antioxidant therapy in HATS was collapsed into two groups receiving either N+S (n= 80) or their PL (n=80). The angiographic data refer to 145 patients of the 146 patients who underwent pre and post therapy angiography (one patient lacked paired glucose/insulin data), n=72 in the N+S group and n=73 in the PL group. The events data refer to 156 patients, since four of the 160 patients lacked paired glucose/insulin data.
Differences in risk factor prevalence between the two treatment groups (N+S and PL) at baseline and during treatment were tested by Pearson’s chi-square test. Within-group changes of baseline and post-treatment clinical characteristics were compared by paired t-test. Differences between the two treatment groups (N+S and PL) in the response to treatment were compared by unpaired t-test. The response to treatment within each of the three different metabolic categories we defined was compared by unpaired t-test.
Risk of primary event reduction in the overall population and in the three different metabolic categories was compared by a chi-square test (Fisher’s exact test).
P<0.05 was considered significant.
Results
Baseline clinical characteristics did not differ significantly between the N+S and the placebo groups (table 1). N+S substantially lowered total cholesterol (−28%), LDL cholesterol (−37%) and triglycerides (−34%), and increased HDL cholesterol levels (+30%). In the placebo group, total and LDL cholesterol fell (−3% and −7%), HDL cholesterol increased to a minor extent (+3%, p=0.002) and triglyceride levels increased slightly (+7%, p=ns).
Table 1.
Main clinical characteristic of patients before and after three years of treatment with placebo or niacin plus simvastatin
| PLACEBOS (n=80) | NIACIN PLUS SIMVASTATIN (n=80) | |||||
|---|---|---|---|---|---|---|
| Baseline | Treatment | p | Baseline | Treatment | p | |
| age (years) | 53.4±8 | ns | 54.0±8 | ns | ||
| sex (M/F) | 71/9 | ns | 69/11 | ns | ||
| glucose (mg/dl) | 101±28 | 101±32 | ns | 104±30 | 107±29 | ns |
| insulin (μU/ml) | 22±10 | 25±12 | ns | 26±15 | 31±20 | 0.006 |
| insulin sensitivity (%) | 49±24 | 46±27 | ns | 47±30 | 40±28 | 0.02 |
| BMI (kg/m2) | 29.4±4 | 29.9±4 | 0.01 | 29.7±5 | 29.9±5 | ns |
| total cholesterol (mg/dl) | 195±28 | 190±22 | ns | 200±35 | 143±29 | <0.001 |
| LDL cholesterol (mg/dl) | 122±26 | 114±22 | 0.001 | 125±32 | 80±22 | <0.001 |
| triglycerides (mg/dl) | 205±104 | 220±144 | ns | 221±101 | 146±87 | <0.001 |
| HDL cholesterol (mg/dl) | 32±4 | 33±5 | 0.001 | 31±5 | 38±7 | <0.001 |
| SBP/DBP (mmHg) | 128±15/80±9 | 131±11/81±8 | ns | 128±15/80±8 | 127±12/78±8 | 0.02§ |
Data indicated as mean±SD. SBP= systolic blood pressure, DBP= diastolic blood pressure, BMI= body mass index, LDL= low density lipoproteins, HDL= high-density lipoproteins.
p refers to paired t-test for treatment vs baseline,
for DBP only
Fasting glucose levels increased slightly (+3% and +1%, p=ns), fasting insulin increased (19%, p=0.004; and 13%, p=ns), and insulin sensitivity decreased (−10%, p=0.02 and −5%, p=ns) in the N+S and the placebo groups, respectively. On treatment, the two groups differed significantly in HDL cholesterol (p<0.0005) and triglyceride levels (p<0.0005), and in diastolic blood pressure values (p<0.05). All other clinical characteristics were comparable. The N+S group showed a minor reduction in blood pressure values (−1% for SBP, p=ns; and −2% for DBP, p=0.02), but no significant changes were observed in the placebo group (+2% for SBP and +1% DBP). BMI remained stable in the N+S group, while it increased minimally, although significantly, in the placebo group (+1%, p=0.01).
At baseline, the proportion of patients fulfilling the NCEP criteria for the MS was comparable: 69% and 71% in the N+S and in the placebo group, respectively (p=ns by χ2 analysis), with a similar relative contribution of each of the risk factors defining the MS (table 2). A history of diabetes was present in 12 and 9 patients in the N+S and in the placebo group, respectively. A history of hypertension was present in 34 and 44 patients in the N+S and in the placebo groups, respectively
Table 2.
Metabolic syndrome components in patients before and after three years of treatment with placebo or niacin plus simvastatin
| PLACEBOS (n=80) | Niacin plus simvastatin (n=80) | |||
|---|---|---|---|---|
| Baseline | Treatment | Baseline | Treatment | |
| High blood pressure (SBP/DBP ≥ 130/85 mmHg or Dg HT) | 53 (66%) | 56 (70%) | 51 (64%) | 47 (59%) |
| High triglycerides (≥150 mg/dl) | 54 (67%) | 49 (61%) | 53 (66%) | 32 (40%)*§ |
| Low HDL cholesterol (<40 mg/dl in M, <50 mg/dl in W) | 80 (100%) | 71 (89%)* | 80 (100%) | 51 (74%)*§ |
| High waist circumference (>102 cm in M, >88 cm in W) | 37 (46%) | 40 (50%) | 43 (54%) | 41 (51%) |
| Dysglycemia (FG ≥ 100 or Dg DM) | 26 (32%) | 26 (32%) | 30 (37%) | 34 (42%) |
| Metabolic syndrome | 57 (71%) | 58 (72%) | 55 (69%) | 45 (56%)*§ |
SBP= systolic blood pressure, DBP= diastolic blood pressure, FG= fasting glucose, M= men, W= women, HDL= high-density lipoproteins, Dg= diagnosis, HT= hypertension, DM= diabetes.
p<0.05, paired t-test for treatment vs baseline,
p<0.05 for Pearson’s chi-square between groups
At the end of the study, there was a significant reduction in the relative frequency of atherogenic dyslipidemia in the N+S group. Low HDL prevalence fell from 100% to 74% and hypertriglyceridemia from 65% to 40%. Patients fulfilling blood pressure criteria for hypertension diminished by −8% (p=ns), while central obesity prevalence did not change. The percentage of patients fulfilling the criteria for impaired fasting glucose (≥100 mg/dl) increased from 37% to 42% (p=ns vs baseline). Overall, the prevalence of the MS was reduced from 69% to 56% with N+S (p<0.05 vs baseline), largely as a result of changes in HDL and triglycerides. In the placebo treated group, reductions in the prevalence of hypertriglyceridemia (−9%) and low HDL (−10%, p<0.05 vs baseline) were less substantial, while waist circumference (+3%) and hypertension (+4%) were non-significantly increased. MS prevalence did not change (+1%).
On treatment, differences between the two groups in the prevalence of single risk factors were significant for high triglycerides (p<0.001), low HDL cholesterol (p<0.005), and the prevalence of the MS (p<0.005).
Subjects were divided into “less insulin resistant” (those above the median for insulin sensitivity) and “more insulin resistant” (those below the median), based on the HOMA2 model. At baseline, 78 patients were less insulin resistant (average insulin sensitivity= 68%) and 78 more insulin resistant (average insulin sensitivity= 29%, data missing from 4 patients).
Subgroup analysis for coronary stenosis
Baseline atherosclerotic burden, as measured by proximal coronary stenosis, was comparable in those with and without metabolic syndrome (36±11% vs 33±8%, respectively), in those who were more and less insulin resistant (35±9% vs 34±10%, respectively), and in those with or without dysglicemia (36±11% vs 33±8%, respectively p= ns for all).
Angiographic stenosis progression during treatment was significantly lower in the N+S group (0.2%) compared to the placebo group (2.8%, p<0.0005 for unpaired t-test). Angiographic stenosis changes were compared according to the presence of MS, more insulin resistance and impaired fasting glycemia/diabetes (figure 1). In the subgroup without the MS, subjects treated with N+S (n=23) showed no change in stenosis severity (Δ%S=−0.006), while those treated with placebo (n=22) showed progression [Δ%S=+2.0, mean difference 2.03% (95% CI 0.40–3.67%), p=0.015]. In the MS group, there was a significantly slower progression of the stenosis in patients treated with N+S (n=49, Δ%S=+0.3) compared to those treated with placebo (n=51, Δ%S=+3.0, mean difference 2.77 [95% CI 0.98–4.56], p=0.003).
Figure 1.
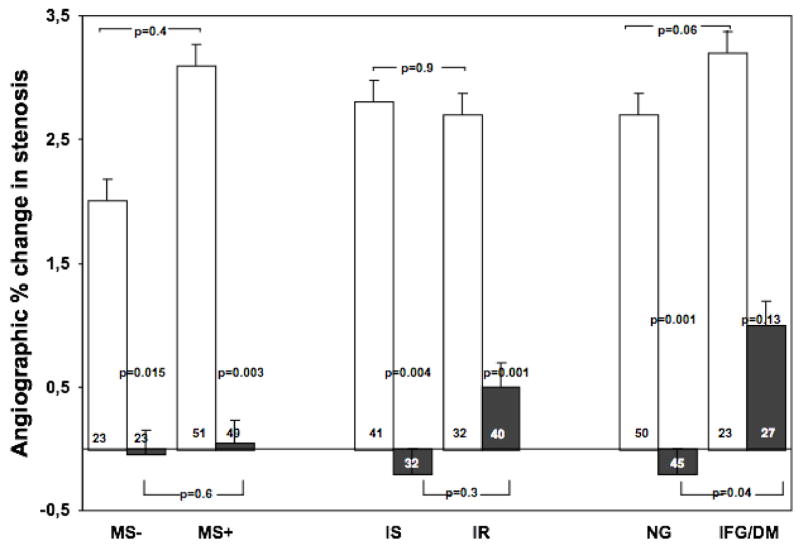
Angiographic change in percent stenosis in patients with or without metabolic syndrome, insulin resistance and glucose intolerance, after three years treatment with PL (white squares) or N+S (black squares), according to their base-line status. Data shown as mean ± SE. MS = metabolic syndrome, IR = (more) insulin resistant, IS = (more) insulin sensitive, NL= normoglycemic, DYSGLY= dysglycemic (impaired fasting glucose or diabetes)
Subjects in the less insulin resistant group (n=32) treated with N+S showed a slight regression in stenosis severity (Δ%S=−0.2), while those treated with placebo (n=41) showed progression (Δ%S=2.8, mean difference 3.08 [95% CI 1.35–4.82], p=0.001). In the more insulin resistant group, there was a significantly slower progression of the stenosis in subjects (n=40) treated with N+S (Δ%S=0.5) compared to those (n=32) treated with placebo (Δ%S=+2.7, mean difference 2.08 [95% CI 0.08–4.09], p=0.045).
In the normoglycemic group, patients treated with N+S (n=45) showed a slight regression in the stenosis amount (Δ%S=−0.2), while those treated with placebo (n=50) showed a progression (Δ%S=+2.7, mean difference 2.92 [95% CI 0.63-4-71], p=0.001). In the dysglycemic group, there was a slower progression of stenosis in patients treated with N+S (n=27, Δ%S=+1.0) compared to those treated with placebo (n=23, %S=+3.2, mean difference 2.04 [95% CI 0.79-4-87], p=0.13).
There is no difference in response to therapy in terms of lipid changes in more or less insulin resistant patients, or in normoglycemic compared to dislgycemic, while patients with metabolic syndrome showed a lesser percentage decrese in TC, TG and LDL compared to those without metabolic syndrome (data not shown).
Clinical event frequency by subgroup analysis
In the overall population, the risk of primary events was reduced by 60% in the N+S treated group compared to the placebo group (8.8% vs 22.5%, p=0.03). The event rate in the placebo group was two fold higher in those classified as having the MS compared to the non-MS group (26% vs 13%, p=ns, fig 2). No events were seen in the non-MS group treated with N+S. Among MS subjects, treatment with N+S reduced events by 47% (from 22.0% to 11.5%, p=0.07), i.e. to an event rate lower than non-MS population that received placebo.
Figure 2.
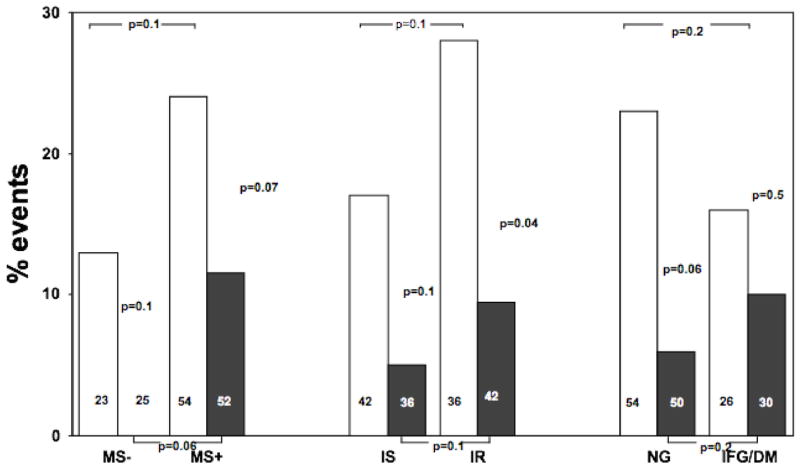
Angiographic change in percent stenosis in patients with or without metabolic syndrome, insulin resistance and glucose intolerance, after three years treatment with PL (white squares) or N+S (black squares), according to their in-treatment status. Data shown as mean ± SE. MS = metabolic syndrome, IR = (more) insulin resistant, IS = (more) insulin sensitive, NL= normoglycemic, DYSGLY= dysglycemic (impaired fasting glucose or diabetes)
The rate of event in the more insulin resistant subgroup was higher, compared to the less insulin resistant subgroup, both in the group treated with placebo (+65%) and in those treated with N+S (+90%, p=ns for both). Therapy with N+S reduced the rate of primary events by a similar amount (65%) in both the less insulin resistant (p=0.1) and in the more insulin resistant group (p=0.04).
In the group treated with placebo, normoglycemic patients surprisingly showed a non significant higher event rate compared to dysglycemic patients (26% vs 15%). The event rate was reduced by N+S therapy in the normoglycemic population (by 76%, p=0.06) and less so in the dysglycemic group (by 13%, p=ns).
Discussion
The results from the original HATS study showed that treatment with N+S had great benefit overall in terms of angiographic progression of stenosis and risk of clinical events. Since the presence of the MS, insulin resistance or dysglycemia have been shown to increase the risk of cardiovascular disease, in this subgroup analysis we investigated whether the presence of 1) MS, 2) greater or lesser degrees of insulin resistance, or 3) impaired fasting glucose/diabetes had an effect on the ability of N+S to influence stenosis progression and clinical events.
The findings in this subgroup analysis provides evidence that the aggressive treatment of atherogenic dyslipidemia with N+S therapy, despite modest worsening of insulin sensitivity beyond its generally low baseline level in this population with established coronary disease, reduces the progression of coronary stenosis in subjects classified as being at even greater risk of developing recurrent manifestations of cardiovascular disease by virtue of having the metabolic syndrome, being insulin resistant or having dysglycemia. Evaluation of the longer term impact of niacin’s effect on insulin resistance and glucose metabolism awaits the results of longer-term clinical trials.
Niacin is a lipid-modifying drug that reduces triglycerides and raises HDL cholesterol levels, leading to a modest reduction in LDL cholesterol levels, particularly small, dense LDL particles (25). Atherogenic dyslipidemia describes the combination of low levels of HDL cholesterol and raised triglycerides, and small dense LDL and HDL particles, which collectively is common in patients with both the MS and type 2 diabetes (6). Thus, niacin should represent an excellent option to treat the atherogenic dyslipidemia that characterizes MS and diabetes. However, niacin has been shown to impair glucose metabolism, increasing insulin resistance and plasma glucose levels. Insulin resistance is believed to increase the risk of developing both cardiovascular disease events (13) and diabetes, although its impact on cardiovascular disease may not be independent of the atherogenic dyslipidemia that is commonly associated with insulin resistance and the MS. Indeed, concerns have been raised about the use of niacin in diabetic patients (26) because of its adverse effects on insulin resistance and blood glucose levels (27). In the HATS subjects, with a high baseline frequency of insulin resistance and a high prevalence of MS, niacin had only a mild detrimental effect on plasma glucose, with a significant increase in fasting insulin and a slight reduction in insulin sensitivity measured using the updated homeostatic model assessment (HOMA2). Previous reports on niacin safety and tolerability among the HATS population showed that glycemic control among diabetic patients returned to pretreatment values after eight months, probably due to better diabetes management – both drugs and diet – in those with higher glucose levels (19).
Type 2 diabetes also is associated with an increased risk of cardiovascular disease (28), with plasma glucose levels being correlated with the development of clinical endpoints in epidemiological studies. However, data from a large clinical trial showed that the extent of treatment of hyperglycemia did not significantly benefit macrovascular complications, while it was very effective in reducing microvascular complications (29). Conversely, a recent meta-analysis that evaluated the clinical benefit of lipid lowering therapy clearly showed that the absolute cardiovascular risk is similarly reduced by lipid lowering therapy in type 2 diabetic populations as much as in non diabetics (30). Since the absolute cardiovascular risk is greater in diabetic subjects than in non diabetics, the potential benefit of lipid therapy is substantial. We therefore questioned whether the beneficial effects of N+S on plasma lipids and lipoproteins could be diminished due to worsening insulin resistance and dysglycemia, particularly since many of the subjects in HATS were insulin resistant, had features of the MS, or were dysglycemic at baseline. Our analysis indicates that subjects showed a beneficial effect from being treated with N+S, whether or not they had MS or were more insulin resistant. The beneficial effect in the group with impaired fasting glucose or diabetes (i.e. the dysglycemic group) was present, but appeared to be somewhat attenuated.
In the overall HATS cohort, major clinical events (including death, myocardial infarction, stroke and need for revascularization) were reduced by 60% compared to placebo over three years of treatment with N+S. The beneficial effect also was detectable when patients were sub-divided according to the presence of MS, insulin resistance, or dysglycemia, although the reduction was not significant in all cases, in part due to the fact that the study was not powered to address these questions.
Because of the unexpectedly higher (though not significantly so) rate of events seen in the placebo-treated normoglycemic population compared to those with dysglycemia, interpreting the effect of N+S on clinical event might be difficult in this particular group of individuals. However, data from the angiographic studies indicate a benefit from treatment with N+S in the subgroup with dysglycemia. Also, the smaller sample size of the dysglicemic group might have hampered the results. Therefore, treating a larger group of subjects might show a benefit in clinical events as well. In the Coronary Drug Project (CDP) niacin reduced the risk of recurrent myocardial infarction at 6 years and of cardiac mortality at 15 years similarly in hyperglycemic (fasting glucose ≥ 100 mg/dl) and normoglycemic patients (31). If anything, the benefits to subjects with diabetes (by current standards ≥ 126 mg/dl) at baseline tended to have greater benefit from niacin at each of these time points. Moreover, a recent post-hoc analysis of the same study showed that patients with and without the MS benefited similarly from therapy with niacin (32). Additional information will be forthcoming from the ongoing AIM-HIGH study, which is evaluating the effect of a statin with and without niacin on cardiovascular end-points.
In summary, in patients with low HDL cholesterol, normal LDL cholesterol levels and coronary artery disease, the presence of either the MS, greater degrees of insulin resistance, or dysglycemia, did not offset the beneficial effect on cardiovascular disease of three years of treatment with N+S, despite a mild impairment of glucose metabolism and increase in insulin resistance. Thus, the benefits obtained by aggressive lipid therapy with N+S appear to justify and overwhelm the negative effects of niacin on insulin sensitivity. The presence of the MS, insulin resistance or diabetes should not discourage the use of niacin in those for whom it otherwise appears indicated. Careful attention should be paid to glucose levels because of their importance in the pathogenesis of microvascular, and possibly macrovascular disease.
Acknowledgments
We thank Dr SJ Robins and H Bloomfield for providing us the equation for the relationship between BMI and waist circumference as derived from the VA-HIT study. Supported in part by the Clinical Nutrition Research Unit at the University of Washington (DK35816).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kahn R, Buse J, Ferrannini E, et al. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care Sep. 2005;28(9):2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 2.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Jama. 2002 Dec 4;288(21):2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 3.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004 Sep 7;110(10):1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 4.Eberly LE, Prineas R, Cohen JD, et al. Metabolic syndrome: risk factor distribution and 18-year mortality in the multiple risk factor intervention trial. Diabetes Care Jan. 2006;29(1):123–130. doi: 10.2337/diacare.29.1.123. [DOI] [PubMed] [Google Scholar]
- 5.Ballantyne CM, Olsson AG, Cook TJ, et al. Influence of low high-density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation. 2001 Dec 18;104(25):3046–3051. doi: 10.1161/hc5001.100624. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM. Hypertriglyceridemia, atherogenic dyslipidemia, and the metabolic syndrome. Am J Cardiol. 1998 Feb 26;81(4A):18B–25B. doi: 10.1016/s0002-9149(98)00033-2. [DOI] [PubMed] [Google Scholar]
- 7.Ballantyne CM, Herd JA, Ferlic LL, et al. Influence of low HDL on progression of coronary artery disease and response to fluvastatin therapy. Circulation. 1999 Feb 16;99(6):736–743. doi: 10.1161/01.cir.99.6.736. [DOI] [PubMed] [Google Scholar]
- 8.Robins SJ, Collins D, Wittes JT, et al. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. Jama. 2001 Mar 28;285(12):1585–1591. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 9.Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation. 2000 Jul 4;102(1):21–27. doi: 10.1161/01.cir.102.1.21. [DOI] [PubMed] [Google Scholar]
- 10.Tenkanen L, Manttari M, Manninen V. Some coronary risk factors related to the insulin resistance syndrome and treatment with gemfibrozil. Experience from the Helsinki Heart Study. Circulation. 1995 Oct 1;92(7):1779–1785. doi: 10.1161/01.cir.92.7.1779. [DOI] [PubMed] [Google Scholar]
- 11.Carr DB, Utzschneider KM, Hull RL, et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes Aug. 2004;53(8):2087–2094. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol Feb. 2004;24(2):e13–18. doi: 10.1161/01.ATV.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 13.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 14.Kamanna VS, Kashyap ML. Mechanism of action of niacin on lipoprotein metabolism. Curr Atheroscler Rep Jan. 2000;2(1):36–46. doi: 10.1007/s11883-000-0093-1. [DOI] [PubMed] [Google Scholar]
- 15.Tenenbaum A, Fisman EZ, Motro M, Adler Y. Atherogenic dyslipidemia in metabolic syndrome and type 2 diabetes: therapeutic options beyond statins. Cardiovasc Diabetol. 2006 Sep 26;5:20. doi: 10.1186/1475-2840-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poynten AM, Gan SK, Kriketos AD, et al. Nicotinic acid-induced insulin resistance is related to increased circulating fatty acids and fat oxidation but not muscle lipid content. Metabolism Jun. 2003;52(6):699–704. doi: 10.1016/s0026-0495(03)00030-1. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM, Vega GL, McGovern ME, et al. Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes: results of the assessment of diabetes control and evaluation of the efficacy of niaspan trial. Arch Intern Med. 2002 Jul 22;162(14):1568–1576. doi: 10.1001/archinte.162.14.1568. [DOI] [PubMed] [Google Scholar]
- 18.Elam MB, Hunninghake DB, Davis KB, et al. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: A randomized trial. Arterial Disease Multiple Intervention Trial. Jama. 2000 Sep 13;284(10):1263–1270. doi: 10.1001/jama.284.10.1263. [DOI] [PubMed] [Google Scholar]
- 19.Zhao XQ, Morse JS, Dowdy AA, et al. Safety and tolerability of simvastatin plus niacin in patients with coronary artery disease and low high-density lipoprotein cholesterol (The HDL Atherosclerosis Treatment Study) Am J Cardiol. 2004 Feb 1;93(3):307–312. doi: 10.1016/j.amjcard.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001 Nov 29;345(22):1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 21.Brown BG, Zhao XQ, Chait A, et al. Lipid altering or antioxidant vitamins for patients with coronary disease and very low HDL cholesterol? The HDL-Atherosclerosis Treatment Study Design. Can J Cardiol Apr. 1998;14(Suppl A):6A–13A. [PubMed] [Google Scholar]
- 22.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, EvaluationAnd Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001 May 16;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 23.Robins SJ, Rubins HB, Faas FH, et al. Insulin resistance and cardiovascular events with low HDL cholesterol: the Veterans Affairs HDL Intervention Trial (VA-HIT) Diabetes Care May. 2003;26(5):1513–1517. doi: 10.2337/diacare.26.5.1513. [DOI] [PubMed] [Google Scholar]
- 24.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care Jun. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 25.Carlson LA. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J Intern Med Aug. 2005;258(2):94–114. doi: 10.1111/j.1365-2796.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- 26.Ito MK. The metabolic syndrome: pathophysiology, clinical relevance, and use of niacin. Ann Pharmacother Feb. 2004;38(2):277–285. doi: 10.1345/aph.1D218. [DOI] [PubMed] [Google Scholar]
- 27.Alvarsson M, Grill V. Impact of nicotinic acid treatment on insulin secretion and insulin sensitivity in low and high insulin responders. Scand J Clin Lab Invest. 1996;56:563–570. doi: 10.3109/00365519609088812. [DOI] [PubMed] [Google Scholar]
- 28.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979 May 11;241(19):2035–8. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 29.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 30.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005 Oct 8;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 31.Canner PL, Furberg CD, Terrin ML, et al. Benefits of niacin by glycemic status in patients with healed myocardial infarction (from the Coronary Drug Project) Am J Cardiol. 2005 Jan 15;95(2):254–257. doi: 10.1016/j.amjcard.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Canner PL, Furberg CD, McGovern ME. Benefits of niacin in patients with versus without the metabolic syndrome and healed myocardial infarction (from the Coronary Drug Project) Am J Cardiol. 2006 Feb 15;97(4):477–479. doi: 10.1016/j.amjcard.2005.08.070. [DOI] [PubMed] [Google Scholar]


