Abstract
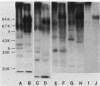
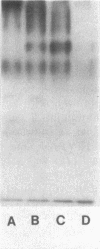
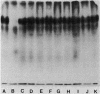
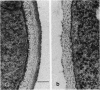
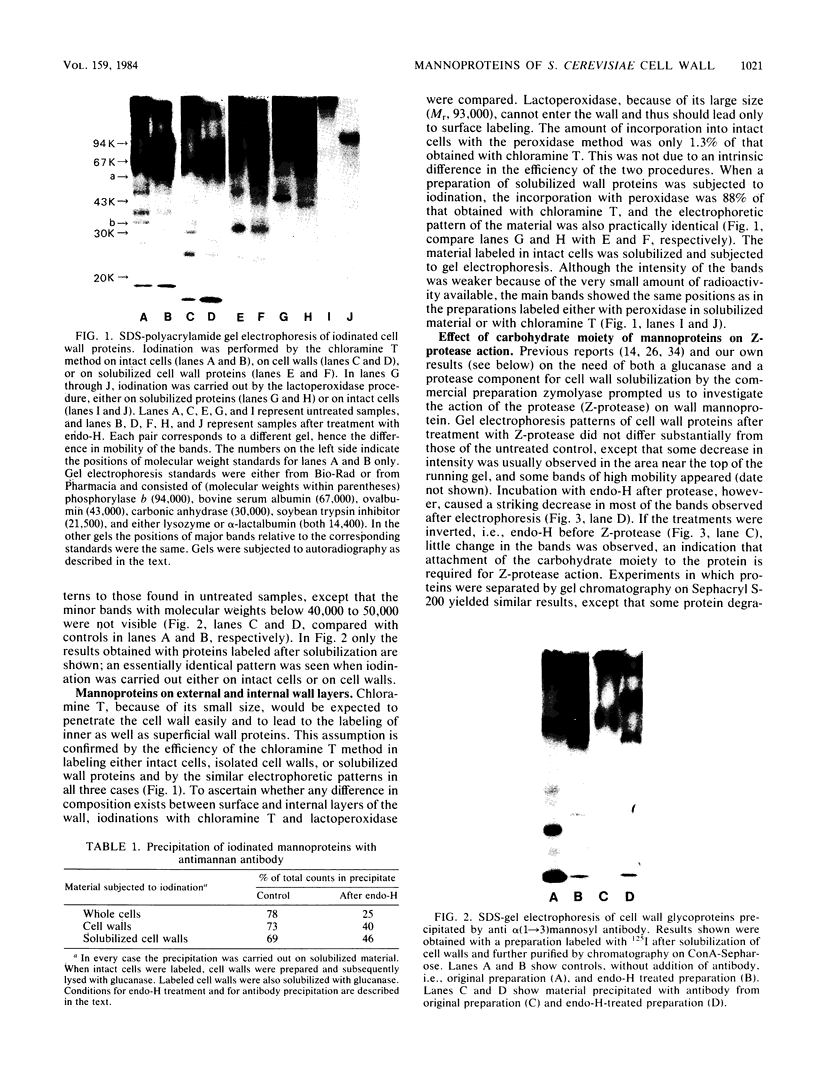
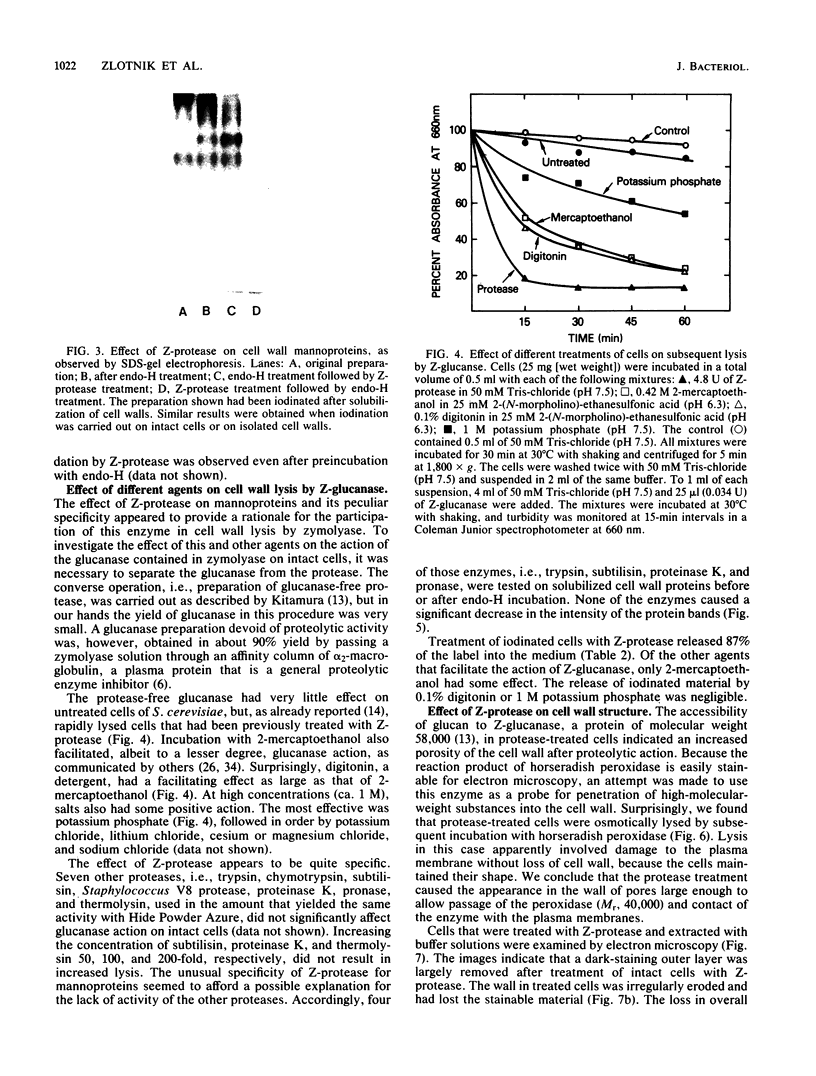
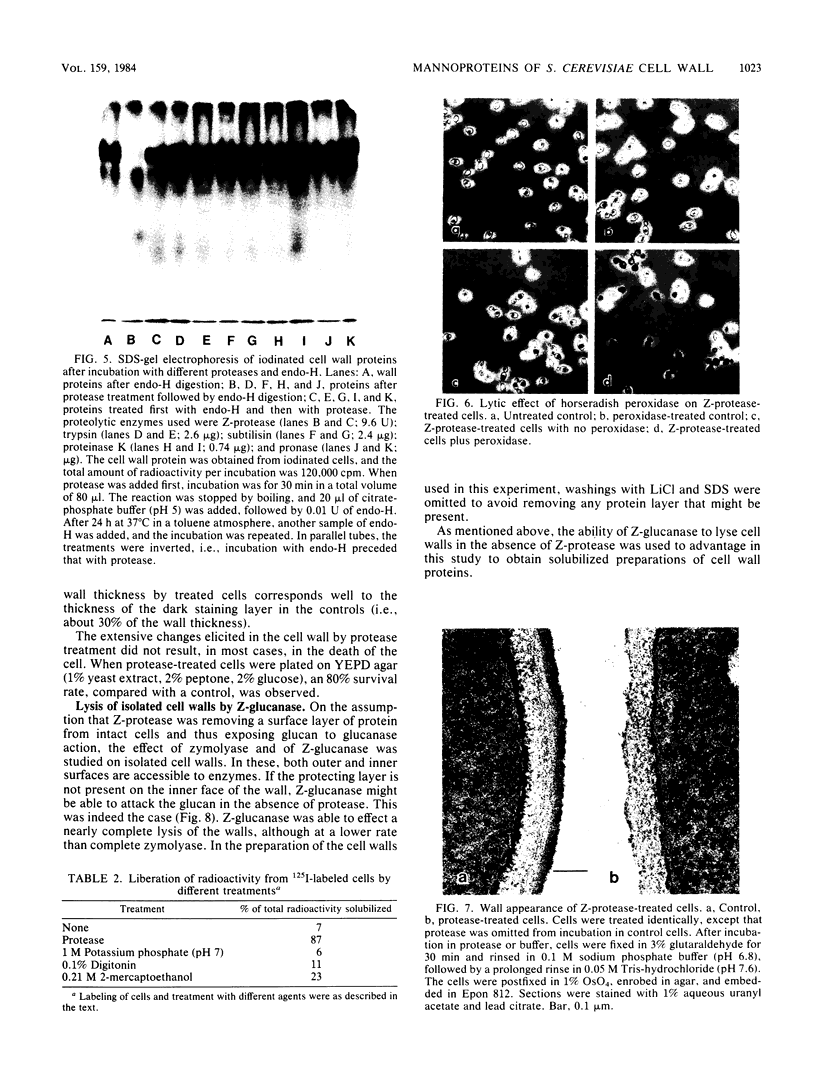
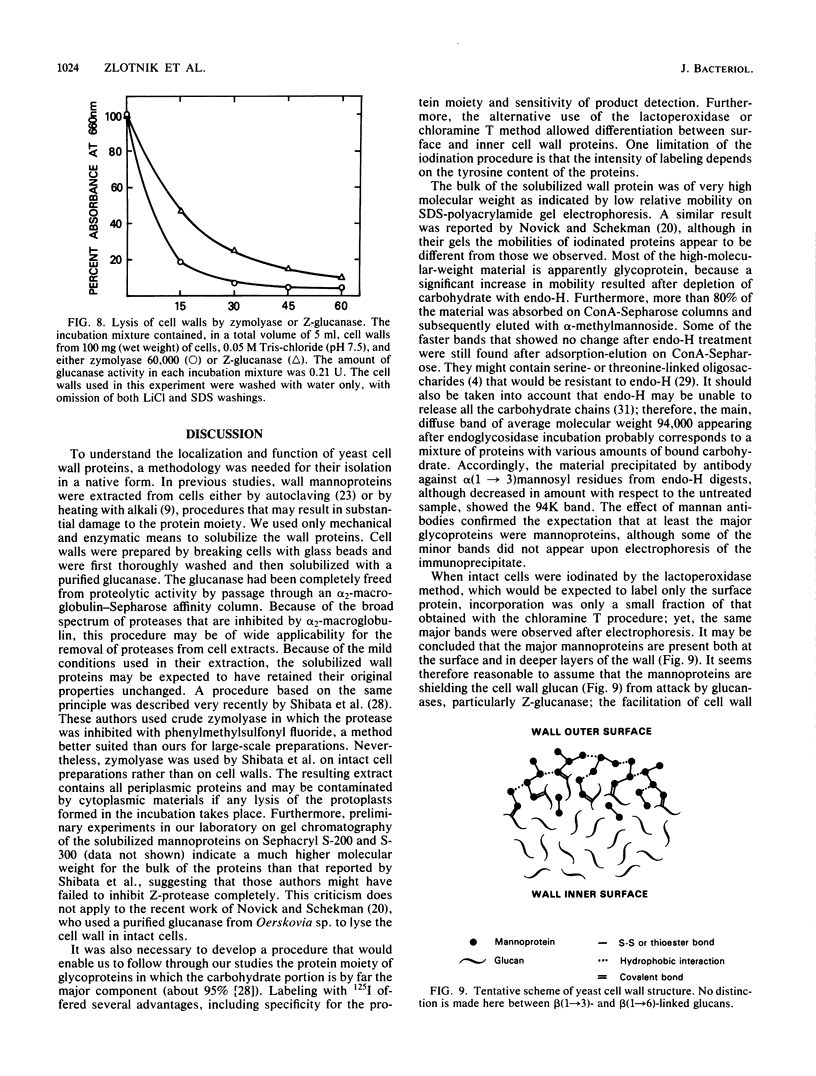
A beta-glucanase (Z-glucanase) from Zymolyase was freed from a protease (Z-protease) by affinity chromatography on alpha 2-macroglobulin-Sepharose columns and used to solubilize proteins from isolated cell walls of Saccharomyces cerevisiae. The cell wall proteins were labeled with 125I and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography. The bulk of the labeled material had very low mobility. Its mannoprotein nature was demonstrated by precipitation with specific antibodies and by conversion to a band with an average molecular weight of 94,000 after incubation with endo-beta-N-acetylglucosaminidase. The intact mannoproteins were hydrolyzed by Z-protease, but were resistant to the enzyme when the carbohydrate was first removed by endo-beta-N-acetylglucosaminidase. In intact cells, lysis of cell walls by Z-glucanase required a previous incubation with z-protease, which led to solubilization of most of the 125I-labeled proteins. Other proteases that did not attack the cell wall mannoproteins were unable to substitute for Z-protease. The specific effect of Z-protease is consistent with the notion that mannoproteins form a surface layer of the cell wall that penetrates the wall to some depth and shields glucans from attack by Z-glucanase. Mannoproteins, however, do not appear to cover the inner face of the cell wall, because isolated cell walls, in contrast to intact cells, were completely solubilized by Z-glucanase in the absence of protease. The function of mannoproteins in determining cell wall porosity was highlighted by the finding that horseradish peroxidase (Mr, 40,000) causes lysis of cells that had been treated with Z-protease. Depletion of mannoproteins by Z-protease also resulted in the disappearance of a darkly stained surface layer of the cell wall, as observed by electron microscopy. Other agents that facilitate cell lysis by Z-glucanase, such as 2-mercaptoethanol, digitonin, and high concentrations of salts, caused little or no solubilization of mannoprotein. We assume that they perturb and loosen the structure of the mannoprotein network, thereby increasing its porosity. The implications of our results for the construction of the yeast cell wall and the anchoring of mannoprotein to the cell are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon J. S., Gordon A. H., Jones D., Taylor I. F., Webley D. M. The separation of beta-glucanases produced by Cytophaga johnsonii and their role in the lysis of yeast cell walls. Biochem J. 1970 Nov;120(1):67–78. doi: 10.1042/bj1200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou C. E. Some aspects of the structure, immunochemistry, and genetic control of yeast mannans. Adv Enzymol Relat Areas Mol Biol. 1974;40(0):239–270. doi: 10.1002/9780470122853.ch6. [DOI] [PubMed] [Google Scholar]
- Ballou C. Structure and biosynthesis of the mannan component of the yeast cell envelope. Adv Microb Physiol. 1976;14(11):93–158. doi: 10.1016/s0065-2911(08)60227-1. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers B., Levin G., Cabib E. Effect of polyoxin D on chitin synthesis and septum formation in Saccharomyces cerevisiae. J Bacteriol. 1974 Aug;119(2):564–575. doi: 10.1128/jb.119.2.564-575.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Roberts R., Bowers B. Synthesis of the yeast cell wall and its regulation. Annu Rev Biochem. 1982;51:763–793. doi: 10.1146/annurev.bi.51.070182.003555. [DOI] [PubMed] [Google Scholar]
- Fleet G. H., Phaff H. J. Lysis of yeast cell walls: glucanases from Bacillus circulans WL-12. J Bacteriol. 1974 Jul;119(1):207–219. doi: 10.1128/jb.119.1.207-219.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurecki T., Kress L. F., Laskowski M., Sr Purification of human plasma alpha 2 macroglobulin and alpha 1 proteinase inhibitor using zinc chelate chromatography. Anal Biochem. 1979 Nov 1;99(2):415–420. doi: 10.1016/s0003-2697(79)80026-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McLellan W. L., Jr, McDaniel L. E., Lampen J. O. Purification of phosphomannanase and its action on the yeast cell wall. J Bacteriol. 1970 Apr;102(1):261–270. doi: 10.1128/jb.102.1.261-270.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Schekman R. Export of major cell surface proteins is blocked in yeast secretory mutants. J Cell Biol. 1983 Feb;96(2):541–547. doi: 10.1083/jcb.96.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. L., Bowers B., Slater M. L., Cabib E. Chitin synthesis and localization in cell division cycle mutants of Saccharomyces cerevisiae. Mol Cell Biol. 1983 May;3(5):922–930. doi: 10.1128/mcb.3.5.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer R., Louden L., Gerhardt P. Porosity of the yeast cell wall and membrane. J Bacteriol. 1974 May;118(2):534–540. doi: 10.1128/jb.118.2.534-540.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. H., Schekman R. Lyticase: endoglucanase and protease activities that act together in yeast cell lysis. J Bacteriol. 1980 May;142(2):414–423. doi: 10.1128/jb.142.2.414-423.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentandreu R., Northcote D. H. The structure of a glycopeptide isolated from the yeast cell wall. Biochem J. 1968 Sep;109(3):419–432. doi: 10.1042/bj1090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata N., Mizugami K., Takano K., Suzuki S. Isolation of mannan-protein complexes from viable cells of Saccharomyces cerevisiae X2180-1A wild type and Saccharomyces cerevisiae X2180-1 A-5 mutant strains by the action of Zymolyase-60,000. J Bacteriol. 1983 Nov;156(2):552–558. doi: 10.1128/jb.156.2.552-558.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Tkacz J. S., Lampen J. O. Wall replication in saccharomyces species: use of fluorescein-conjugated concanavalin A to reveal the site of mannan insertion. J Gen Microbiol. 1972 Sep;72(2):243–247. doi: 10.1099/00221287-72-2-243. [DOI] [PubMed] [Google Scholar]
- Trimble R. B., Maley F., Chu F. K. GlycoProtein biosynthesis in yeast. protein conformation affects processing of high mannose oligosaccharides on carboxypeptidase Y and invertase. J Biol Chem. 1983 Feb 25;258(4):2562–2567. [PubMed] [Google Scholar]
- Ulane R. E., Cabib E. The activating system of chitin synthetase from Saccharomyces cerevisiae. Purification and properties of the activating factor. J Biol Chem. 1976 Jun 10;251(11):3367–3374. [PubMed] [Google Scholar]
- Virca G. D., Travis J., Hall P. K., Roberts R. C. Purification of human alpha-2-macroglobulin by chromatography on Cibacron Blue Sepharose. Anal Biochem. 1978 Aug 15;89(1):274–278. doi: 10.1016/0003-2697(78)90750-9. [DOI] [PubMed] [Google Scholar]
- Vrsanská M., Biely P., Krátký Z. Enzymes of the yeast lytic system produced by Arthrobacter GJM-1 bacterium and their role in the lysis of yeast cell walls. Z Allg Mikrobiol. 1977;17(6):465–480. doi: 10.1002/jobm.3630170608. [DOI] [PubMed] [Google Scholar]
- Yphantis D. A., Dainko J. L., Schlenk F. Effect of some proteins on the yeast cell membrane. J Bacteriol. 1967 Nov;94(5):1509–1515. doi: 10.1128/jb.94.5.1509-1515.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]