Abstract
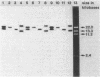
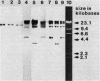
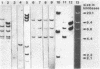
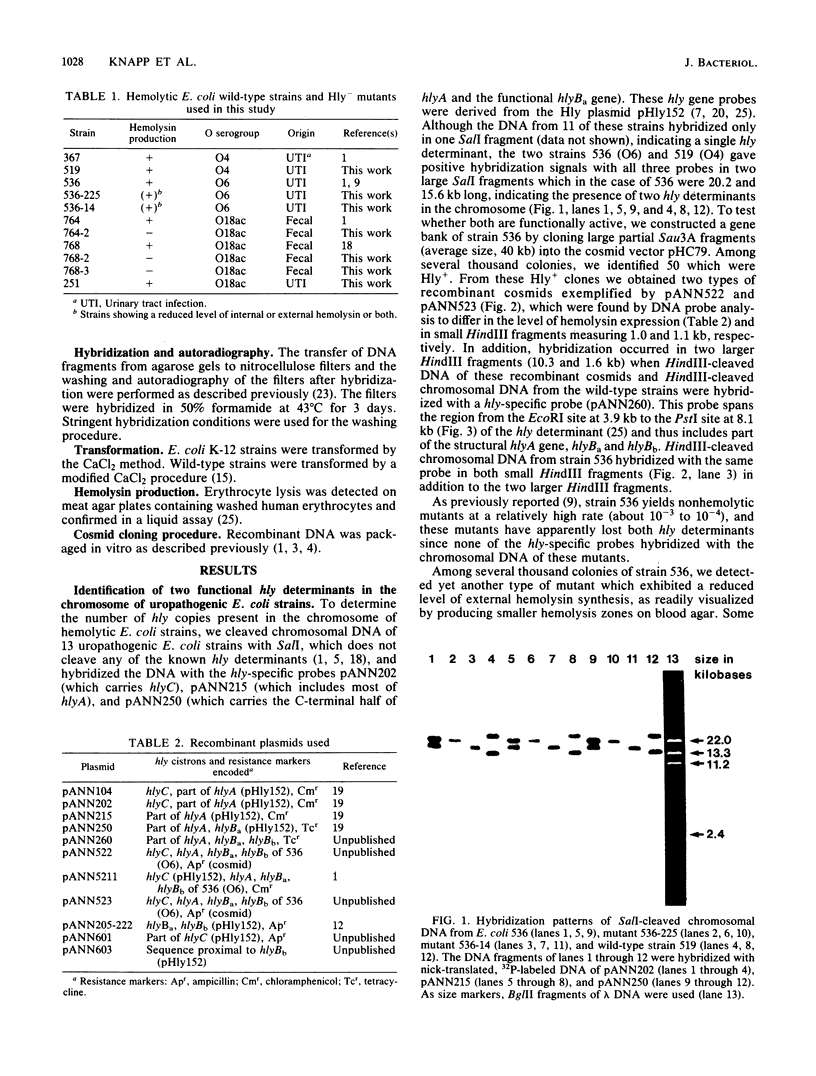
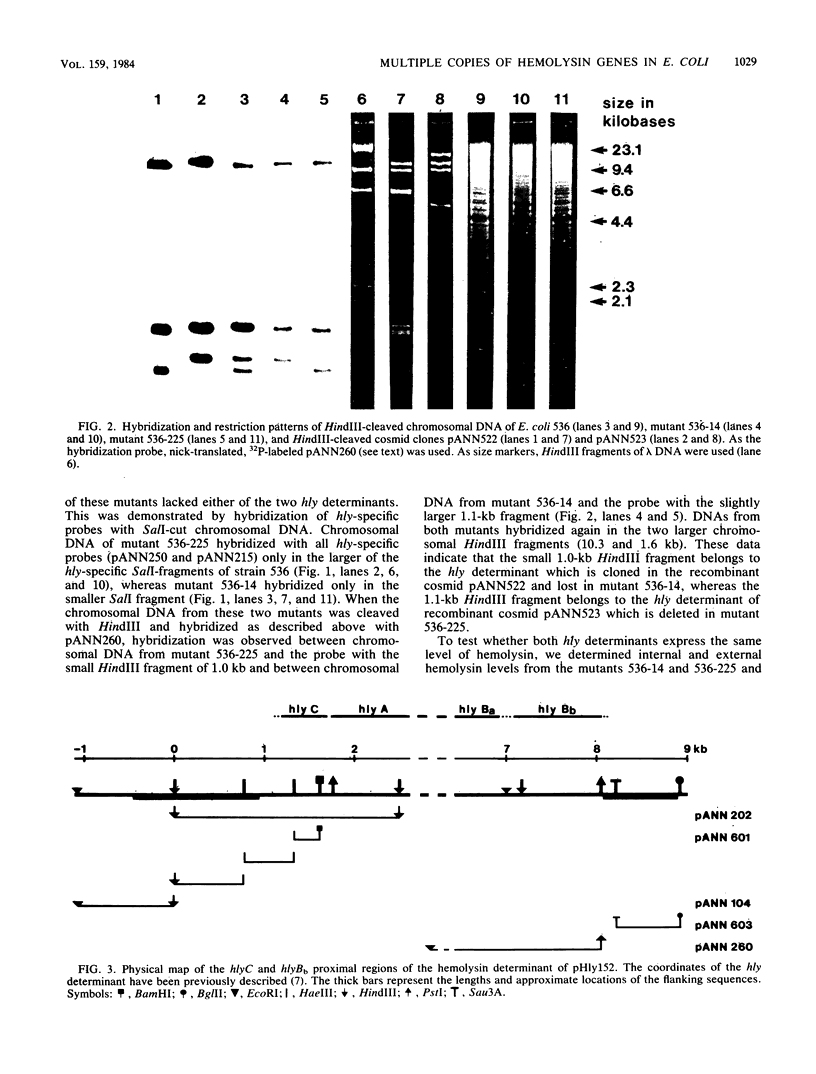
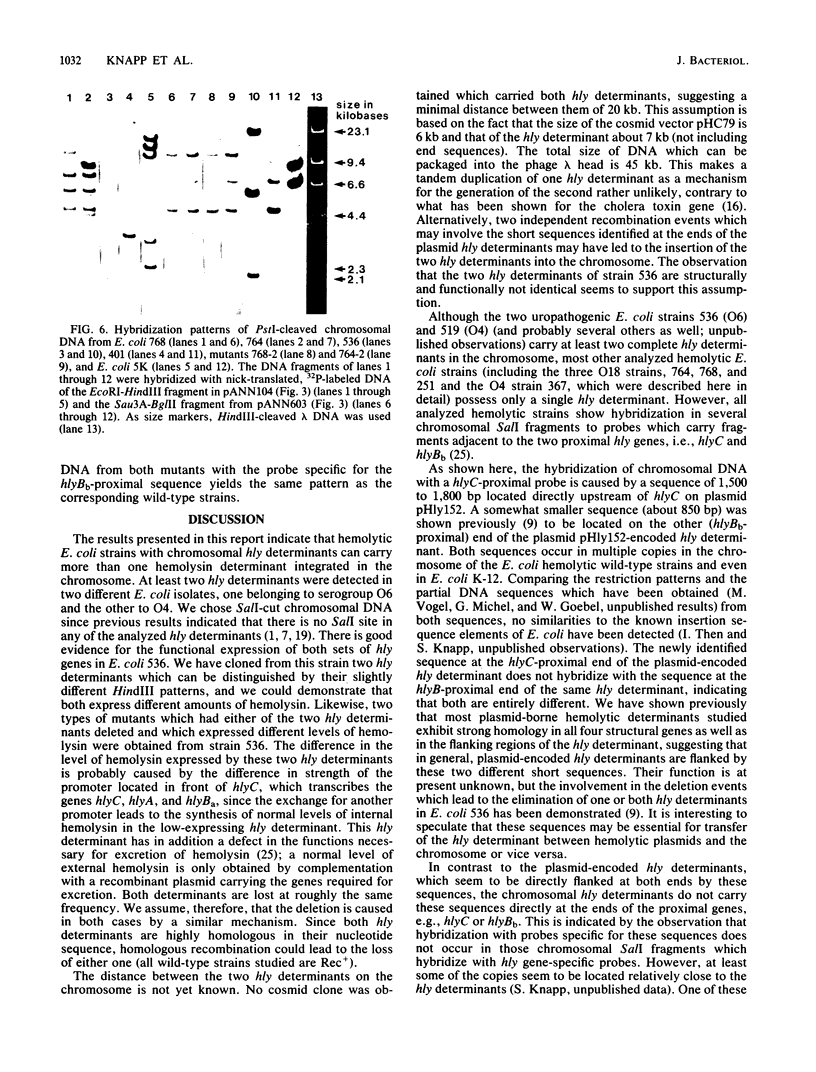
The O6 serogroup Escherichia coli strain 536 carries two hemolysin (hly) determinants integrated into the chromosome. The two hly determinants are not completely identical, either functionally or structurally, as demonstrated by spontaneous deletion mutants carrying only one of them and by cloning each of the two determinants separately into cosmid vectors. Each hly determinant is independently deleted at a frequency of 10(-4), leading to variants which exhibit similar levels of internal hemolysin but different amounts of secreted hemolysin. The two hly determinants were also identified in the O4 E. coli strain 519. The three E. coli strains 251, 764, and 768, which belong to the serogroup O18, and the O4 strain 367 harbor a single chromosomal hly determinant, as demonstrated by hybridization with hly-gene-specific probes. However, a hybridization probe derived from a sequence adjacent to the hlyC-proximal end of the plasmid pHly 152-encoded hly determinant hybridizes with several additional chromosomal bands in hemolytic O18 and O6 E. coli strains and even in E. coli K-12. The size of the probe causing the multiple hybridization suggests a 1,500- to 1,800-base pair sequence directly flanking hlyC. Spontaneous hemolysin-negative mutants were isolated from strains 764 and 768, which had lost the entire hly determinant but retained all copies of the hlyC-associated sequence.2+.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger H., Hacker J., Juarez A., Hughes C., Goebel W. Cloning of the chromosomal determinants encoding hemolysin production and mannose-resistant hemagglutination in Escherichia coli. J Bacteriol. 1982 Dec;152(3):1241–1247. doi: 10.1128/jb.152.3.1241-1247.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Brüning H. J. Plasmids useable as gene-cloning vectors in an in vitro packaging by coliphage lambda: "cosmids". Gene. 1978 Oct;4(2):85–107. doi: 10.1016/0378-1119(78)90023-9. [DOI] [PubMed] [Google Scholar]
- Collins J., Hohn B. Cosmids: a type of plasmid gene-cloning vector that is packageable in vitro in bacteriophage lambda heads. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4242–4246. doi: 10.1073/pnas.75.9.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden C. S., Eriksson B., Hanson L. A. Adhesion of Escherichia coli to human uroepithelial cells in vitro. Infect Immun. 1977 Dec;18(3):767–774. doi: 10.1128/iai.18.3.767-774.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Young L. S., Pitt J. Hemagglutination typing of Escherichia coli: definition of seven hemagglutination types. J Clin Microbiol. 1980 Aug;12(2):235–242. doi: 10.1128/jcm.12.2.235-242.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W., Hedgpeth J. Cloning and functional characterization of the plasmid-encoded hemolysin determinant of Escherichia coli. J Bacteriol. 1982 Sep;151(3):1290–1298. doi: 10.1128/jb.151.3.1290-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted J., Bennett P. M., Higginson S., Richmond M. H. Regional preference of insertion of Tn501 and Tn802 into RP1 and its derivatives. Mol Gen Genet. 1978 Nov 9;166(3):313–320. doi: 10.1007/BF00267624. [DOI] [PubMed] [Google Scholar]
- Hacker J., Knapp S., Goebel W. Spontaneous deletions and flanking regions of the chromosomally inherited hemolysin determinant of an Escherichia coli O6 strain. J Bacteriol. 1983 Jun;154(3):1145–1152. doi: 10.1128/jb.154.3.1145-1152.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Schröter G., Schrettenbrunner A., Hughes C., Goebel W. Hemolytic Escherichia coli strains in the human fecal flora as potential urinary pathogens. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 May;254(3):370–378. [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C., Hacker J., Roberts A., Goebel W. Hemolysin production as a virulence marker in symptomatic and asymptomatic urinary tract infections caused by Escherichia coli. Infect Immun. 1983 Feb;39(2):546–551. doi: 10.1128/iai.39.2.546-551.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull S. I., Hull R. A., Minshew B. H., Falkow S. Genetics of hemolysin of Escherichia coli. J Bacteriol. 1982 Aug;151(2):1006–1012. doi: 10.1128/jb.151.2.1006-1012.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtlein M., Schiessl S., Wagner W., Rdest U., Kreft J., Goebel W. Transport of hemolysin by Escherichia coli. J Cell Biochem. 1983;22(2):87–97. doi: 10.1002/jcb.240220203. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983 Nov;35(1):253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- Minshew B. H., Jorgensen J., Counts G. W., Falkow S. Association of hemolysin production, hemagglutination of human erythrocytes, and virulence for chicken embryos of extraintestinal Escherichia coli isolates. Infect Immun. 1978 Apr;20(1):50–54. doi: 10.1128/iai.20.1.50-54.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D., Hughes C., Goebel W. Relationship between plasmid and chromosomal hemolysin determinants of Escherichia coli. J Bacteriol. 1983 Feb;153(2):846–851. doi: 10.1128/jb.153.2.846-851.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noegel A., Rdest U., Goebel W. Determination of the functions of hemolytic plasmid pHly152 of Escherichia coli. J Bacteriol. 1981 Jan;145(1):233–247. doi: 10.1128/jb.145.1.233-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noegel A., Rdest U., Springer W., Goebel W. Plasmid cistrons controlling synthesis and excretion of the exotoxin alpha-haemolysin of Escherichia coli. Mol Gen Genet. 1979 Oct 1;175(3):343–350. doi: 10.1007/BF00397234. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Halls S. The transmissible nature of the genetic factor in Escherichia coli that controls haemolysin production. J Gen Microbiol. 1967 Apr;47(1):153–161. doi: 10.1099/00221287-47-1-153. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wagner W., Vogel M., Goebel W. Transport of hemolysin across the outer membrane of Escherichia coli requires two functions. J Bacteriol. 1983 Apr;154(1):200–210. doi: 10.1128/jb.154.1.200-210.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabala J. C., de la Cruz F., Ortiz J. M. Several copies of the same insertion sequence are present in alpha-hemolytic plasmids belonging to four different incompatibility groups. J Bacteriol. 1982 Jul;151(1):472–476. doi: 10.1128/jb.151.1.472-476.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz F., Müller D., Ortiz J. M., Goebel W. Hemolysis determinant common to Escherichia coli hemolytic plasmids of different incompatibility groups. J Bacteriol. 1980 Aug;143(2):825–833. doi: 10.1128/jb.143.2.825-833.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]