Abstract
Focal cerebral infarction (stroke) due to unilateral occlusion of the middle cerebral artery in mature rats produces deficits in sensorimotor function of the contralateral limbs that recover partially over time. We found that biweekly intracisternal injection of basic fibroblast growth factor (bFGF; 0.5 μg/injection), a potent neurotrophic polypeptide, markedly enhanced recovery of sensorimotor function of the contralateral limbs during the first month after stroke without apparent adverse side effects. Immunostaining for growth-associated protein 43 (GAP-43), a molecular marker of axonal sprouting, showed a selective increase in GAP-43 immunoreactivity in the intact sensorimotor cortex contralateral to cerebral infarcts following bFGF treatment. These results show that bFGF treatment can enhance functional recovery after stroke, and that the mechanism may include stimulation of neuronal sprouting in the intact brain.
Keywords: growth-associated protein 43, cerebral ischemia, growth factors
Stroke, a disease that affects more than 500,000 Americans annually, is commonly due to thromboembolic occlusion of a cerebral artery, resulting in the focal death of brain tissue (cerebral infarction) (1). Stroke patients may suffer disturbances of motor strength and coordination, sensory discrimination, visual function, speech, memory, or other intellectual abilities. Although recovery is often incomplete, partial recovery often occurs in the weeks to months following stroke (2).
The precise anatomical, cellular, and molecular mechanisms of functional recovery following stroke remain unknown. Although damaged neurons and axons in the mammalian brain do not regenerate, local neuronal sprouting and synaptic reorganization do occur that are likely to contribute to functional recovery. For example, in rodents and primates, recovery of sensorimotor function following unilateral cerebrocortical injury or stroke is accompanied by new neuronal sprouting and synapse formation in adjacent brain regions surrounding focal infarcts and in regions homologous to infarcted cortex in the contralateral hemisphere (3–6). In recovering human stroke patients, functional imaging techniques demonstrate increased cerebral blood flow and metabolism in intact brain regions surrounding focal cerebral infarcts, most likely reflecting increased neuronal activity in these regions (7).
Basic fibroblast growth factor (bFGF) is an 18-kDa protein with potent trophic effects on brain neurons, glia, and endothelial cells (8). In particular, bFGF supports the survival and outgrowth of a wide variety of brain neurons both in vitro and in vivo (8–10). In previous studies, we showed that bFGF, administered intracerebrally or intravenously within the first few hours after the onset of ischemia, reduced the volume of focal cerebral infarcts in models of unilateral middle cerebral artery (MCA) occlusion in both rats and cats (11–13). Intravenous bFGF crosses the damaged blood brain barrier to enter ischemic brain tissue, where it appears to reduce infarct size by direct protection of vulnerable cells at the margins (“penumbra”) of focal infarcts (12, 14). In subsequent studies (15) we found that if bFGF was administered intracerebrally (intracisternally) at later times (≥24 h) after infarction, infarct volume was not reduced, but animals recovered more rapidly and to a greater extent on tests of sensorimotor function of the contralateral limbs. The intracisternal route was chosen for these studies to permit access of bFGF to the intact brain, which may contribute to recovery (3–6). Possible mechanisms of bFGF-induced enhancement of functional recovery include protection against the “retrograde” death of distant neurons, as well as potentiation of new neuronal sprouting and synapse formation (3, 4, 15, 16). At the dose of intracisternal bFGF used in these initial studies (8 μg total), weight loss was also observed in some animals (15).
In the current study, we used a lower dose of bFGF to enhance functional recovery without apparent adverse effects. Moreover, to explore possible mechanisms of bFGF action, we examined the expression a molecular marker of new axonal growth, namely the neuronal growth-associated protein 43 (GAP-43). GAP-43 is a phosphoprotein component of the neuronal membrane and growth cone that is selectively up-regulated during new axonal growth in both the peripheral and central nervous systems (17–21). GAP-43 has been used as a reliable marker of new axonal growth during brain development, and following brain injury or ischemia (5, 20, 21). We examined GAP-43 immunoreactivity (IR) in animals with focal infarcts receiving, or not receiving, intracisternal bFGF.
METHODS
Stroke Surgery and Intracisternal Injections.
Focal infarcts were made in the right dorsolateral cerebral cortex and underlying striatum of mature male Sprague–Dawley rats by occlusion of the proximal MCA, as described (15, 22). Briefly, male Sprague–Dawley rats (275–325 g; Charles River Breeding Laboratories) were anesthetized with 2% halothane in 70% NO2/30% O2. Body temperature was maintained at 37°C. The proximal right MCA was electrocoagulated from just proximal to the olfactory tract to the inferior cerebral vein, and was then transected. Sham-operated animals were prepared in similar fashion, but without occlusion of the MCA. The tail artery was cannulated for monitoring of arterial blood gases and glucose levels which were measured once just before surgery, and once at the end of surgery, and these values were not different among stroke or sham animals ultimately receiving bFGF or vehicle. Cefazolin sodium (40 mg/kg, i.p.) was administered on the day before and just after stroke surgery. Following surgery, animals were placed in individual cages. Animals were weighed on the day before surgery and every other day thereafter.
Recombinant human bFGF (obtained from Scios, Mt. View, CA) was diluted in vehicle containing 0.9% saline with 100 μg/ml of BSA (Boehringer Mannheim, catalogue no. 711454). For intracisternal injections, animals were re-anesthetized with 2% halothane in 70% NO2/30% O2 and placed in a stereotaxic frame. Rats received bFGF in vehicle (0.5 μg in 50 μl) or vehicle alone (50 μl) by percutaneous injection into the cisterna magna, beginning at 24 h after infarction and continuing twice weekly for 4 weeks, as described (15). Animals were assigned bFGF or vehicle treatment in random fashion.
Behavioral Testing.
Animals were handled for 10 min each day for 3 days before stroke surgery. Beginning on the day before surgery and continuing every other day thereafter for 1 month, animals were examined using three standard tests to assess sensorimotor function in the limbs as well as vestibulomotor function, as described (15, 23, 24). Specifically, the forelimb placing test measures sensorimotor function in each forelimb as the animal places the limb on a tabletop in response to visual, tactile, and proprioceptive stimuli (total score = 0–10; 10 = maximally impaired) (23). Similarly, the hindlimb placing test measures sensorimotor function of the hindlimb as the animal places it on a tabletop in response to tactile and proprioceptive stimuli (total score = 0–6; 6 = maximally impaired) (23). The modified beam balance test examines vestibulomotor activity as the animal balances on a narrow beam (30 × 1.3 cm) for 60 sec (score range = 1–7; 7 = maximally impaired) (24).
In addition to these standard behavioral tests, we also measured the spontaneous motor activity of the forelimbs, as described (3, 4). Animals were placed in a narrow glass cylinder (16.5 × 25 cm) and videotaped for 10 min on the day before stroke surgery and at weekly intervals thereafter. Videotapes were then scored independently by two experienced observers (5 min per rat per day) for the number of spontaneous movements made by each forelimb to initiate rearing, to land on or to move laterally along the wall of the cylinder, or to land on the floor after rearing. The scores of the two observers were averaged, and the mean number of spontaneous movements of each limb was expressed as a percentage of the total to compute the “spontaneous limb use index” for each forelimb. This index has been used as a sensitive and reliable test of sensorimotor impairment and subsequent recovery following unilateral cerebrocortical injury (3, 4). The mean number of total forelimb movements analyzed per session was 74 ± 5; this number was not different among bFGF- or vehicle-treated animals.
Histology and Infarct Volume Determination.
One month (on day 31) after stroke, animals were deeply anesthetized with sodium pentobarbital and perfused transcardially with 10% buffered formalin. Brains were removed, postfixed in formalin, dehydrated, and embedded in paraffin. Coronal sections (5 μm) were cut on a microtome, mounted onto glass slides, and stained with hematoxylin/eosin (H&E). The area of cerebral infarcts on each of seven slices (+4.7, +2.7, +0.7, −1.3, −3.3, −5.3, and −7.3 mm compared with bregma) was determined using a computer-interfaced imaging system (Bioquant, Nashville, TN) using the “indirect method” (area of the intact contralateral hemisphere − area of the intact ipsilateral hemisphere) to correct for brain shrinkage during processing (25). Infarct volume was then expressed as a percentage of the intact contralateral hemispheric volume. Volumes of infarction in the cortex and striatum were also determined separately using these same methods. H&E-stained sections were also examined for gross histological changes, such as hemorrhage, abscess, or tumor formation.
GAP-43 Immunohistochemistry.
To explore potential mechanisms of action of bFGF, an additional set of vehicle- and bFGF-treated animals with stroke and corresponding sham-operated controls were prepared, and their brains were immunostained for GAP-43. Animals were killed at 3, 7, or 14 days after surgery by transcardial perfusion fixation with normal saline followed by 2% paraformaldehyde, 0.01 M sodium-m-periodate, and 0.075 M l-lysine monohydrochloride in 0.1 M sodium phosphate buffer (pH 7.4). Brains were removed, postfixed, and cut on a vibratome at 40 μm, and the sections were cryoprotected. Free floating sections were successively incubated in 20% normal goat serum, a mouse monoclonal antibody to GAP-43 (1:500, clone 91E12; Boehringer Mannheim), and biotinylated horse anti-mouse IgG adsorbed against rat IgG (45 μl/10 ml; Vector Laboratories), and the reaction product was visualized using standard avidin-biotin horseradish peroxidase/diaminobenzidine methods (Vectastain ABC Kit; Vector Laboratories). Sections were then mounted onto glass slides, air dried, immersed in gradient ethanol, and coverslipped. Brain sections from all animals at each time point were immunostained simultaneously. Control sections processed without primary antibody showed no specific staining.
Immunostaining data were examined on two standard coronal sections (“anterior” and “posterior”) through cerebral infarcts (+0.2 and −2.8 mm compared with bregma, respectively), and relative changes in the intensity and extent of GAP-43 IR were quantified using a computer-interfaced imaging system (Bioquant) by two different methods. Adjacent brain sections, stained with H&E, were used to identify the extent of infarcts. The optical density (OD) of a region of reliably low GAP-43 IR (the corpus callosum) was considered the “background” value for each section. For method 1, all brain regions showing an OD of 1.5 times or greater compared with background were identified and highlighted (see Figs. 2 and 3). The area (in mm2) of highlighted regions in the dorsolateral sensorimotor cortex was determined for each slice and averaged among animals in each group. For method 2, specific regions of dorsolateral sensorimotor cortex were identified using a published standard rat brain atlas (26). On anterior brain sections, these included the medial peri-infarct cortex (≤1 mm from the infarct border) in the ipsilateral hemisphere, and frontal cortex areas 1 and 2 (FR1 and -2) and forelimb area of cortex (FL) regions in both hemispheres (Fig. 2). On posterior sections, these included the medial peri-infarct region in the ipsilateral hemisphere, as well as FR1 and -2 and hindlimb area of cortex (HL) regions bilaterally (see Fig. 3). The OD was determined for each region on each section and normalized to background. For each method, data in sham/vehicle-treated and sham/bFGF-treated animals were not different, so that these values were pooled in the analysis. Data in all groups were expressed as ratios compared with stroke/vehicle-treated animals.
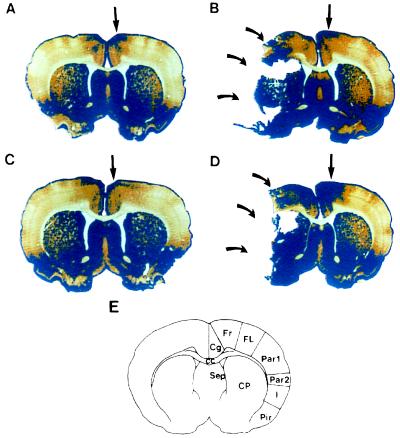
Figure 2.
Changes in GAP-43 IR in anterior brain sections following bFGF treatment. Representative sections are from sham/vehicle-treated animals (A), stroke/vehicle-treated animals (B), sham/bFGF-treated animals (C), and stroke/bFGF-treated animals (D). Animals were killed 3 days after surgery. The blue color shows regions of “high” GAP-43 IR—i.e., regions with OD 1.5 times or greater compared with a standard region (the corpus callosum) on each slice. Curved arrows point to cerebral infarcts. (E) Schematic diagram of brain regions. Cg, cinglulate cortex; FR, frontal cortex, areas 1 and 2; FL, forelimb area; Par1 and -2, parietal cortex, areas 1 and 2; I, insular cortex; Pir, piriform cortex; CC, corpus callosum; Sep, septal nucleus; CP, caudoputamen. In all sham-operated animals (A and C) and in stroke/vehicle-treated animals (B), high GAP-43 IR is restricted to regions of Cg and FR1 and -2 (straight arrows). However, in stroke/bFGF-treated animals (D), the region of high GAP-43 IR spreads to involve the entire FR1 and -2 and part of FL.
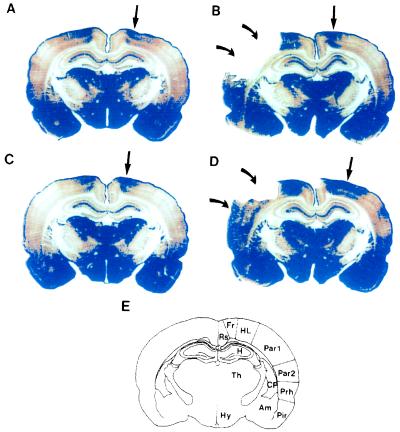
Figure 3.
Changes in GAP-43 IR in posterior brain sections following bFGF treatment. Representative sections are from sham/vehicle-treated animals (A), stroke/vehicle-treated animals (B), sham/bFGF-treated animals (C), and stroke/bFGF-treated animals (D). Animals were killed 3 days after surgery. The blue color shows regions of “high” GAP-43 IR (see legend to Fig. 2). Curved arrows point to cerebral infarcts. In B, all necrotic tissue has fallen off the slide; in D, some infarcted tissue remains (lower curved arrow), but is necrotic as determined by H&E staining of adjacent sections. (E) Schematic diagram of brain regions. Rs, retrosplenial cortex; FR, frontal cortex, areas 1 and 2; HL, hindlimb area; Par1 and -2, parietal cortex, areas 1 and 2; Prh, perirhinal cortex; Pir, piriform cortex; Am, amygdala; CP, caudoputamen; CC, corpus callosum; H, hippocampus; Th, thalamus; Hy, hypothalamus. In all sham-operated animals (A and C) and in stroke/vehicle-treated animals (B), high GAP-43 IR is restricted to HL in the dorsolateral cortex (straight arrows). However, in stroke/bFGF-treated animals (D), the region of high GAP-43 IR spreads to involve Par1.
Data Analysis.
All intracisternal injections, behavioral testing, and subsequent histological analyses were done by investigators blinded to the treatment assignment of each animal. Data were expressed as means ± SEM, and were analyzed by one- or two-way ANOVA followed by appropriate pairwise post hoc tests with correction for multiple comparisons.
RESULTS
As described previously (15, 22), infarcts produced by occlusion of the proximal MCA involved the dorsolateral cerebral cortex and underlying subcortical structures in the ipsilateral hemisphere (see Figs. 2 and 3). Specifically, infarcts involved regions of cortex controlling sensorimotor function of the contralateral limbs [frontal cortex areas 1, 2, and 3 (FR1, -2, -3); forelimb area of cortex (FL); hindlimb area of cortex (HL); and parietal cortex areas 1 and 2 (Par1 and -2)], as well as insular cortex (I); temporal cortex, areas 1 and 3 (Te1 and -3); occipital cortex, area 2, lateral (Oc2L); and caudate putamen (CP; see Figs. 2 and 3) (26). At 1 month after stroke, infarct volumes in the cortex, striatum, and total infarct volumes were not different between bFGF- and vehicle-treated animals (Table 1). These data are consistent with previous results showing that bFGF administration starting at one day after ischemia is beyond the “therapeutic window” during which bFGF can reduce infarct size (15, 16, 27). There was no evidence of hemorrhage, abscess, or tumor formation in any brain examined.
Table 1.
Infarct volume ± SEM (expressed as a percentage of the volume of the intact contralateral hemisphere, cortex, or striatum) in stroke/vehicle-treated (n = 6) vs. stroke/bFGF-treated (n = 8) animals at 31 days after surgery
| Treatment group | Vehicle | bFGF |
|---|---|---|
| Total | 29.1 ± 2.2 | 29.2 ± 2.0 |
| Cortex | 38.1 ± 3.2 | 37.1 ± 2.2 |
| Striatum | 68.1 ± 3.3 | 69.1 ± 2.1 |
All animals survived during the first month after stroke. Overall, animals lost weight (25 ± 2 g, n = 14) during the first day and then gained weight steadily (132 ± 6 g) for the next 30 days after stroke. There were no differences in body weight between stroke/bFGF- and stroke/vehicle-treated animals [F(1) = 2.94; P = n.s.).
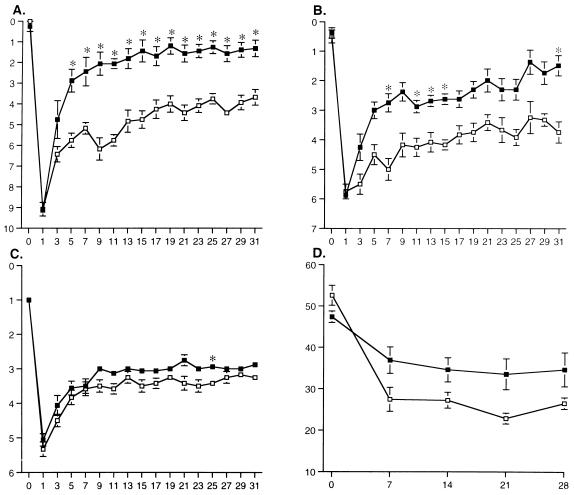
As reported previously (15), animals with unilateral cerebral infarcts showed marked disturbances in placing of the contralateral (left) forelimb and hindlimb as well as in beam balance (Fig. 1 A–C). No deficits were observed in placing of the ipsilateral (right) limbs in animals with stroke or in any behaviors tested in sham-operated animals (data not shown). Stroke/vehicle-treated animals showed partial recovery of contralateral forelimb and hindlimb placing and of beam balance behavior during the first month after stroke (Fig. 1 A–C). By contrast, stroke/bFGF-treated animals showed marked enhancement in recovery of contralateral forelimb and hindlimb placing, and a lesser, but still significant, degree of enhancement of recovery of beam balance behavior compared with stroke/vehicle controls (Fig. 1 A–C). In addition to these standard behavioral tests, we also examined spontaneous motor activity of the forelimbs after stroke, as described (3, 4). As expected, before infarction, spontaneous use of the contralateral forepaw was approximately 50% for all animals (Fig. 1D). However, by one week after infarction, and continuing for the next month, spontaneous use of the contralateral forelimb was greater in stroke/bFGF- than in stroke/vehicle-treated animals (Fig. 1D).
Figure 1.
Behavioral recovery following intracisternal bFGF treatment. □, Stroke/vehicle-treated rats (n = 6); ▪, stroke/bFGF-treated rats (n = 8). X-axes indicate time (days) after stroke surgery. Y-axes indicate scores on the individual behavioral tests as described in the text. Data are mean ± SEM and were analyzed by two-way repeated measures ANOVA (treatment × time) followed by appropriate pairwise two-tailed t tests with Bonferroni correction. Asterisks indicate individual values in bFGF-treated animals that differ from the corresponding values in vehicle-treated animals by P < 0.05. (A) Forelimb placing: treatment effect, F(1) = 32.7, P = 0.0001; time effect, F(16) = 62.6, P = 0.0001; interaction, F(16) = 5.7, P = 0.0001. (B) Hindlimb placing: treatment effect, F(1) = 34.6, P = 0.0001; time effect, F(16) = 31.7, P = 0.0001; interaction, F(16) = 2.3, P = 0.005. (C) Beam balance: treatment effect, F(1) = 15.9, P = 0.002; time effect, F(16) = 71.5, P = 0.0001; interaction, F(16) = 0.78, P = n.s. (D) Spontaneous limb use: treatment effect, F(1) = 6.72, P = 0.02; time effect, F(4) = 23.16, P = 0.0001; interaction, F(4) = 3.09, P = 0.03.
To examine possible mechanisms of enhancement of behavioral recovery by bFGF, we killed animals at various time points (3, 7, or 14 days) after surgery and immunostained their brains for GAP-43, a molecular marker of new axonal growth (17–21). At all time points examined, the pattern of GAP-43 IR in sham-operated animals receiving either bFGF or vehicle was similar to that described previously for the intact mature rat brain (ref. 28; see Figs. 2 and 3). Specifically, GAP-43 IR was relatively high in the ventrolateral cerebral cortex and striatum, hypothalamus, parts of thalamus, amygdala, and hippocampal formation (Figs. 2 and 3). GAP-43 IR was relatively low in the dorsolateral sensorimotor cortex, except for parts of the FR1 and -2 cortex in anterior brain sections and HL cortex in posterior sections (Figs. 2 and 3).
Following stroke, increased GAP-43 IR was found in peri-infarct cortex in the ipsilateral hemisphere, peaking at 3 days after ischemia, which is consistent with previous reports (5). There were no obvious differences in GAP-43 IR seen in the ipsilateral peri-infarct cortex between stroke/vehicle- and stroke/bFGF-treated animals. Moreover, no differences were found in the contralateral hemisphere of stroke/vehicle-treated compared with sham/vehicle- or sham/bFGF-treated animals (Figs. 2 and 3). However, in stroke/bFGF-treated animals, a marked increase in GAP-43 IR was found within the contralateral sensorimotor cortex. Specifically, regions of high GAP-43 IR were larger, spreading ventrally to involve the entire FR1 and -2 cortex and part of FL cortex in “anterior” brain sections, and to involve Par1 cortex in “posterior” brain sections (Figs. 2 and 3).
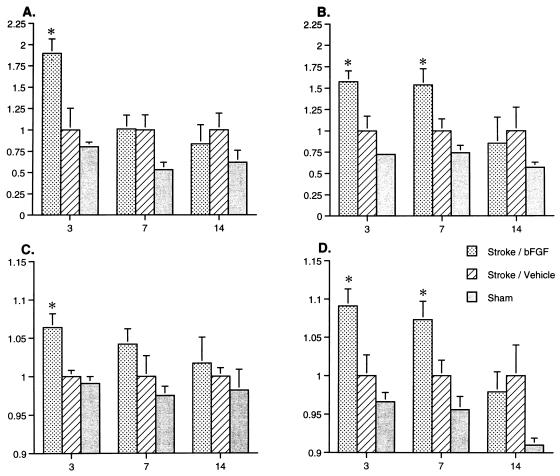
These immunohistochemical changes were quantified by image analysis in two ways. Both the total area of high GAP-43 IR, as well as the relative GAP-43 IR in specific regions of the contralateral sensorimotor cortex (including FL and HL regions), were significantly higher in stroke/bFGF-treated animals compared with the other groups (Fig. 4). These changes were maximal at 3 days after infarction (Fig. 4). There was also a trend toward higher GAP-43 in the ipsilateral peri-infarct cortex of stroke/bFGF-treated animals compared with stroke/vehicle-treated animals 3 days after infarction, but these changes did not reach significance (data not shown).
Figure 4.
Quantitative changes in GAP-43 IR in contralateral dorsolateral cortex following bFGF treatment. Data are mean ± SEM and represent relative changes in GAP-43 IR in sham-operated, stroke/vehicle-treated, and stroke/bFGF-treated rats killed at 3, 7, or 14 days after surgery (n = 4–8 animals each point). X-axes indicate time (days) after stroke surgery. Y-axes indicate relative changes in GAP-43 IR, normalized to stroke/vehicle-treated animals. Data were analyzed by two-way ANOVA (treatment × time) followed by appropriate post hoc Fisher’s protected least significant difference tests. Values in sham/vehicle-treated and sham/bFGF-treated animals were not different and were pooled in the analysis. Asterisks indicate specific values in stroke/bFGF-treated animals differ from those in corresponding stroke/vehicle-treated animals by P < 0.05. (A) Relative area of “high” GAP-43 IR in the contralateral (left) dorsolateral cortex in anterior brain sections. Treatment effect: F(2) = 10.36, P = 0.0003; time effect: F(2) = 5.32, P = 0.01; interaction: F(4) = 2.96, P = 0.03. (B) Relative area of high GAP-43 IR in the contralateral (left) dorsolateral cortex in posterior brain sections. Treatment effect: F(2) = 11.15, P = 0.0002; time effect: F(2) = 2.81, P = n.s.; interaction: F(4) = 1.48, P = n.s. (C) Relative intensity of GAP-43 IR in the forelimb area (FL) of the contralateral (left) cortex in anterior brain sections. Treatment effect: F(2) = 5.91, P = 0.006; time effect: F(2) = 0.51, P = n.s.; interaction: F(4) = 0.334, P = n.s. (D) Relative intensity of GAP-43 IR in the hindlimb area (HL) of the contralateral (left) cortex in posterior brain sections. Treatment effect: F(2) = 15.11, P = 0.0001; time effect: F(2) = 4.47, P = 0.02; interaction: F(4) = 1.47, P = n.s.
DISCUSSION
In summary, we found that repeated intracisternal injections of bFGF, beginning 1 day after infarction, enhanced recovery of sensorimotor function of the contralateral limbs. Improved recovery on standard behavioral tests (forelimb and hindlimb placing, and beam balance) was paralleled by enhanced spontaneous use of the contralateral forelimb. There was no difference in infarct volume between bFGF- and vehicle-treated animals. Intracisternal bFGF treatment was associated with a selective increase in GAP-43 IR in the intact sensorimotor cortex contralateral to infarcts.
These results are consistent with previous studies (15) in which we found that a higher dose of intracisternal bFGF (8 μg total) also enhanced sensorimotor recovery of the contralateral limbs, as assessed by limb placing and beam balance tests. However, at this higher dose, we also found weight loss among some bFGF-treated animals (15). At the bFGF dose used in the current study (4 μg total), we found an equivalent degree of enhancement of neurological recovery compared with the higher dose without weight loss. In both studies, histological examination of brains 1 month after surgery showed no apparent adverse morphological effects of bFGF treatment, including hemorrhage, abscess, or tumor formation.
bFGF supports the survival of a wide variety of CNS neurons in vitro and protects these neurons against a number of toxins and insults, including anoxia, hypoglycemia, excitatory amino acids, free radicals, excess intracellular calcium, and nitric oxide (8, 9, 29, 30). The mechanism of protection appears to involve direct receptor-mediated induction of signal transduction pathways leading to the expression of “neuroprotective” genes and their products (30). bFGF also enhances process outgrowth and branching from cultured neurons; these processes are predominantly axons (9, 10). If bFGF is administered intracerebrally or systemically within a few hours after the onset of focal cerebral ischemia, infarct size is reduced, most likely due to direct protection of cells at the borders (“penumbra”) of infarcts (11–14). Systemically administered bFGF crosses the damaged blood brain barrier and is thus available to ischemic brain tissue (12). In the current study, we found no reduction of infarct volume when bFGF was administered intracisternally beginning 1 day after infarction. These results are consistent with previous reports showing that one day is beyond the “therapeutic window” during which intravenous, intraventricular, or intracisternal bFGF can reduce infarct size (15, 16, 27). Nonetheless, we found enhanced neurological recovery among bFGF-treated animals.
In an effort to explore the possible mechanisms of enhancement of behavioral recovery by bFGF, we immunostained brains for the neuronal protein GAP-43. GAP-43 is a membrane-associated phosphoprotein that is transported from the neuronal cell body to the axon terminal and growth cone (17–21). Although the precise function of GAP-43 is unclear, this protein appears to play an important role in axonal outgrowth, branching, and path finding (17–21, 31). Thus, whereas GAP-43 is expressed constitutively in selected regions of the mature rat brain, its expression is markedly up-regulated in parallel with new axonal growth occurring during brain development and with new axonal sprouting occurring after brain injury or ischemia (17–21).
The current findings of selectively increased GAP-43 IR in the contralateral sensorimotor cortex of stroke/bFGF-treated animals is consistent with enhanced axonal sprouting in this region. Axonal sprouting is likely to be accompanied, in turn, by new dendritic growth and synapse formation that are likely to play an important role in functional recovery. Indeed, previous studies have shown increased dendritic sprouting in layer V of the contralateral homotopic cortex following unilateral electrolytic injury to the rat sensorimotor cortex (3, 4). Moreover, both new dendritic growth and functional recovery of the affected forelimb can be inhibited by immobilization of the intact forelimb (4). These data suggest that “use-dependent” neuronal sprouting and synapse formation in the intact contralateral cortex contributes to functional recovery following unilateral cortical injury (4). The mechanisms of recovery may be direct, through uncrossed projections from intact cortex to impaired limbs, or indirect, through “learning” of other pathways to impaired limbs, enabled by enhanced performance of intact limbs. Interestingly, we did not find increased GAP-43 IR in the contralateral cortex of stroke/vehicle-treated animals, in spite of the fact that axonal sprouting was likely occurring in these animals as well, suggesting that changes in GAP-43 may have been below the threshold of detection given our methods. On the other hand, consistent with previous reports (5), we did find increased GAP-43 IR in the ipsilateral peri-infarct cortex of both stroke/vehicle-treated and stroke/bFGF-treated animals, indicating that neuronal sprouting in the ipsilateral hemisphere also occurs and may play a role in recovery. There was a trend toward increased GAP-43 IR in the ipsilateral peri-infarct cortex of stroke/bFGF-treated compared with stroke/vehicle-treated rats, but these data did not reach significance as did findings on the contralateral side.
The current data supporting the participation of the intact contralateral hemisphere in functional recovery in rats are paralleled by data suggesting that the intact hemisphere may play an important role in functional recovery in human stroke patients. Recent studies using functional imaging (positron-emission tomography and functional MRI) techniques show increased blood flow and metabolic activity in the intact cerebral hemisphere, occurring in parallel with functional recovery following unilateral stroke (7). Clinical experience indicates that stroke patients with good functional recovery may experience a recurrence of their original deficits if a new stroke occurs in the previously intact contralateral hemisphere (32). Our current findings lend support to the concept that the intact contralateral hemisphere participates in recovery, and suggest that intracisternal bFGF may represent a potential new treatment to enhance functional recovery from stroke.
Acknowledgments
This work was supported by Grant NS10828 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and by Scios, Inc. (Mt. View, CA).
ABBREVIATIONS
- bFGF
basic fibroblast growth factor
- GAP-43
growth-associated protein 43
- IR
immunoreactivity
- MCA
middle cerebral artery
- H&E
hematoxylin/eosin
- HL
hindlimb
- FR
frontal cortex
- FL
forelimb
- Par
parietal
References
- 1.Barnett H J M, Mohr J P, Stein B M, Yatsu F M. Stroke: Pathophysiology, Diagnosis, and Management. New York: Churchill Livingstone; 1992. [Google Scholar]
- 2.Dumbovy M L, Bach-y-Rita P. In: Functional Recovery in Neurological Disease. Waxman S, editor. New York: Raven; 1988. pp. 265–276. [Google Scholar]
- 3.Jones T A, Schallert T. Brain Res. 1992;581:156–160. doi: 10.1016/0006-8993(92)90356-e. [DOI] [PubMed] [Google Scholar]
- 4.Jones T A, Schallert T. J Neurosci. 1994;14:2140–2152. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stroemer R P, Kent T A, Hulsebosch C E. Stroke. 1995;26:2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- 6.Nudo R J, Wise B M, SiFuentes F, Milliken G W. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 7.Weiller C, Chollet F, Friston K J, Wise R J, Frackowiak R S. Ann Neurol. 1992;31:463–472. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- 8.Baird A. Curr Opin Neurobiol. 1994;4:78–86. doi: 10.1016/0959-4388(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 9.Walicke P A. J Neurosci. 1988;8:2618–2627. doi: 10.1523/JNEUROSCI.08-07-02618.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel M N, McNamara J O. Neuroscience. 1995;69:763–770. doi: 10.1016/0306-4522(95)00281-m. [DOI] [PubMed] [Google Scholar]
- 11.Koketsu N, Berlove D J, Moskowitz M A, Kowall N W, Caday C G, Finklestein S P. Ann Neurol. 1994;35:451–457. doi: 10.1002/ana.410350413. [DOI] [PubMed] [Google Scholar]
- 12.Fisher M, Meadows M-E, Do T, Weise J, Trubetskoy V, Charette M, Finklestein S P. J Cereb Blood Flow Metab. 1995;15:953–959. doi: 10.1038/jcbfm.1995.121. [DOI] [PubMed] [Google Scholar]
- 13.Bethel A, Kirsch J R, Koehler R C, Finklestein S P, Traystman R J. Stroke. 1997;28:609–615. doi: 10.1161/01.str.28.3.609. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z, Chen K, Huang P L, Finklestein S P, Moskowitz M A. Am J Physiol. 1996;272:H1401–H1405. doi: 10.1152/ajpheart.1997.272.3.H1401. [DOI] [PubMed] [Google Scholar]
- 15.Kawamata T, Alexis N E, Dietrich W D, Finklestein S P. J Cereb Blood Flow Metab. 1996;16:542–547. doi: 10.1097/00004647-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Yamada K, Kinoshita A, Kohmura E, Sakaguchi T, Taguchi J, Kataoka K, Hayakawa T. J Cereb Blood Flow Metab. 1991;11:472–478. doi: 10.1038/jcbfm.1991.90. [DOI] [PubMed] [Google Scholar]
- 17.Skene J H P. Annu Rev Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- 18.Aigner L, Arber S, Kapfhammer J P, Laux T, Schneider C, Botteri F, Brenner H-R, Caroni P. Cell. 1995;83:269–278. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 19.Woolf C J, Reynolds M L, Molander C, O’Brien C, Lindsay R M, Benowitz L I. Neuroscience. 1990;34:465–478. doi: 10.1016/0306-4522(90)90155-w. [DOI] [PubMed] [Google Scholar]
- 20.Benowitz L I, Rodriguez W, Neve R L. Mol Brain Res. 1990;8:17–23. doi: 10.1016/0169-328x(90)90004-w. [DOI] [PubMed] [Google Scholar]
- 21.Vaudano E, Campbell G, Anderson P N, Davies A P, Woolhead C, Schreyer D J, Lieberman A R. J Neurosci. 1995;15:3594–3611. doi: 10.1523/JNEUROSCI.15-05-03594.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamura A, Graham D I, McCulloch J, Teasdale G M. J Cereb Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- 23.DeRyck M, van Reempts J, Duytschaever H, van Deuren B, Clincke G. Brain Res. 1992;573:44–60. doi: 10.1016/0006-8993(92)90112-m. [DOI] [PubMed] [Google Scholar]
- 24.Clifton G L, Jiang J Y, Lyeth B G, Jenkins L W, Hamm R J, Hayes R L. J Cereb Blood Flow Metab. 1991;11:114–121. doi: 10.1038/jcbfm.1991.13. [DOI] [PubMed] [Google Scholar]
- 25.Swanson R A, Morton M T, Tsao-Wu G, Savalos R A, Davidson C, Sharp F R. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 27.Tenjin H, Anderson R E, Meyer F B. J Neurol Sci. 1995;128:66–70. doi: 10.1016/0022-510x(94)00208-6. [DOI] [PubMed] [Google Scholar]
- 28.Benowitz L I, Apostolides P J, Perrone-Bizzozero N, Finklestein S P, Zwiers H. J Neurosci. 1988;8:339–352. doi: 10.1523/JNEUROSCI.08-01-00339.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattson M P, Scheff S W. J Neurotrauma. 1994;11:3–33. doi: 10.1089/neu.1994.11.3. [DOI] [PubMed] [Google Scholar]
- 30.Mattson M P, Barger S W. In: Cerebrovascular Diseases: The 19th Princeton Stroke Conference. Moskowitz M A, Caplan L R, editors. Newton, MA: Butterworth Heinemann; 1995. pp. 271–290. [Google Scholar]
- 31.Yankner B A, Benowitz L I, Villa-Komaroff L, Neve R L. Mol Brain Res. 1990;7:39–44. doi: 10.1016/0169-328x(90)90071-k. [DOI] [PubMed] [Google Scholar]
- 32.Fisher C M. Can J Neurol Sci. 1992;19:57–63. [PubMed] [Google Scholar]