Abstract
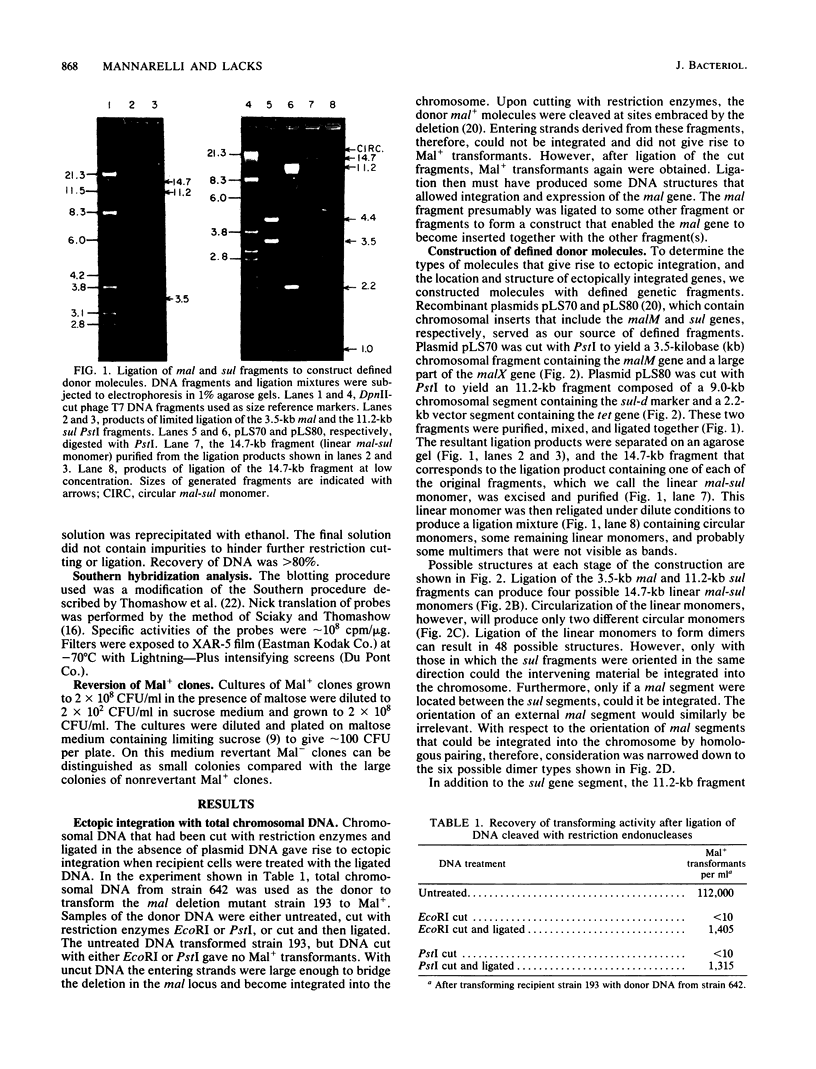
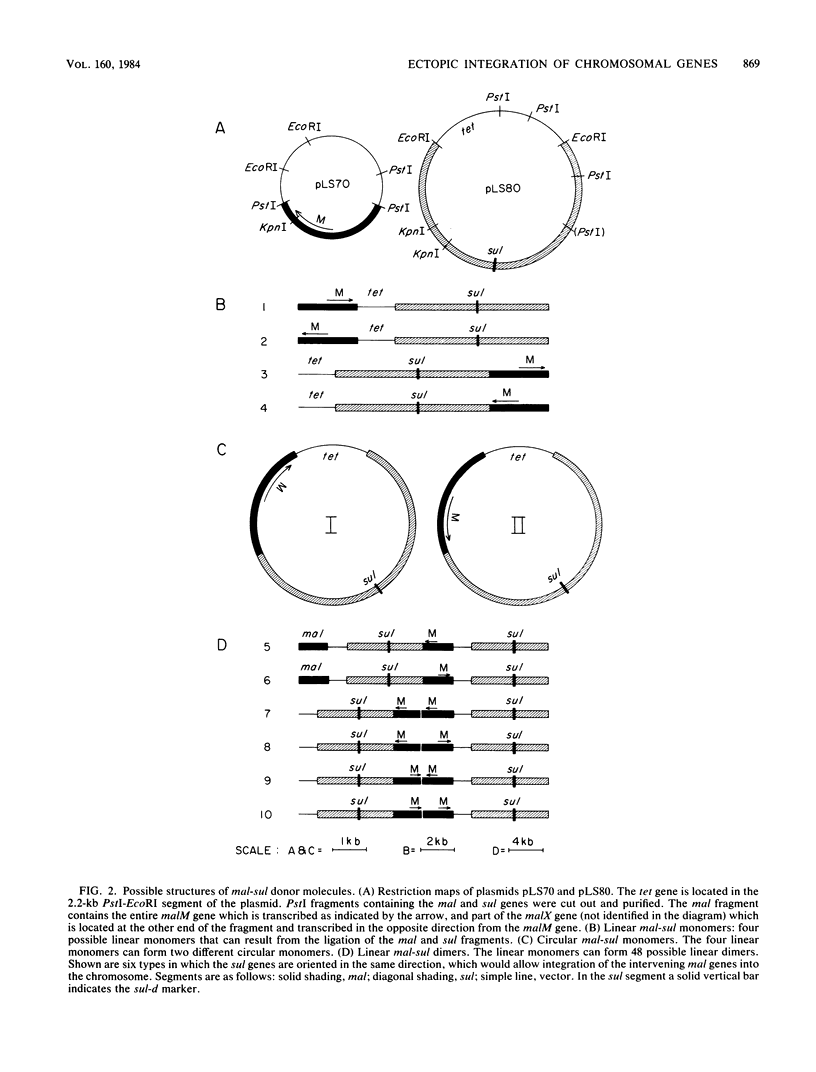
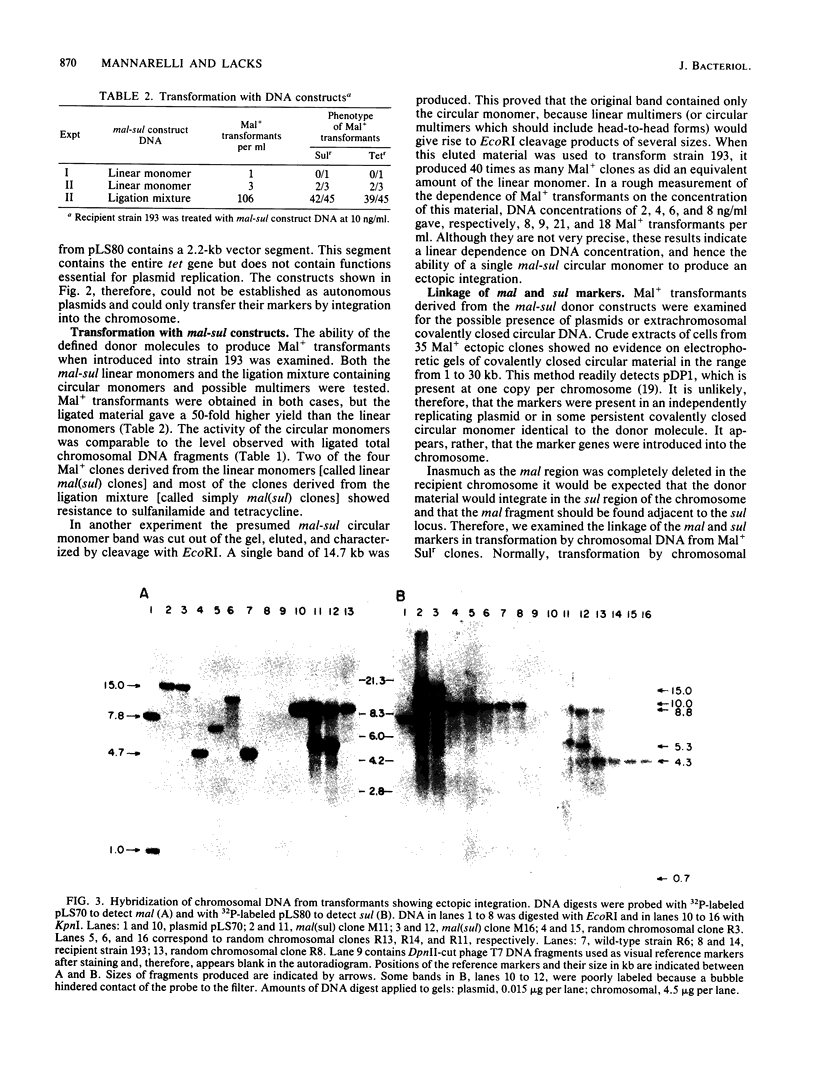
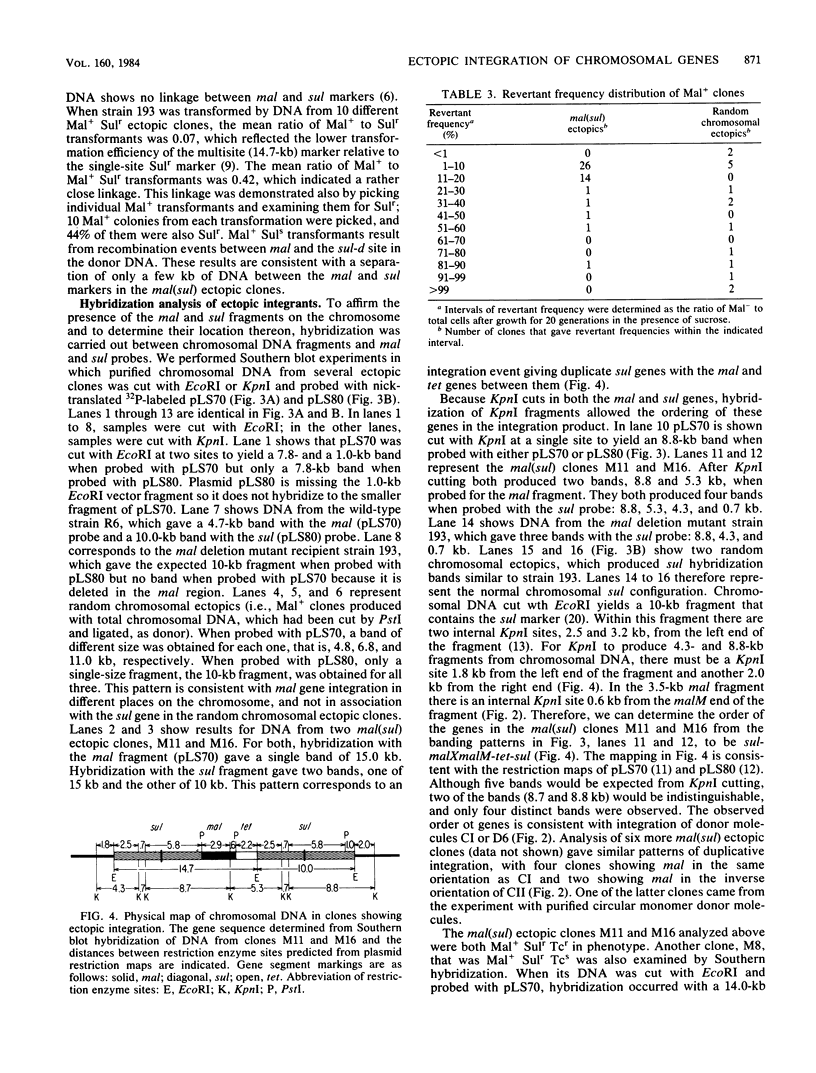
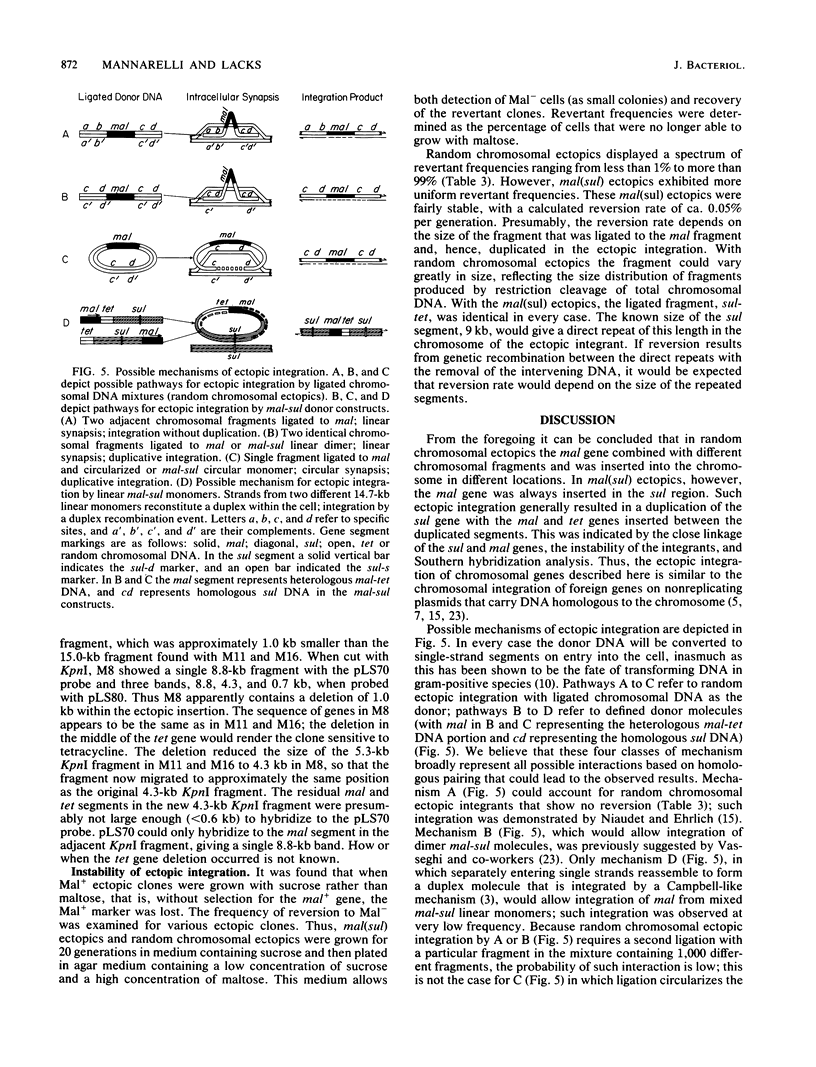
When a DNA fragment containing a marker gene was ligated to random chromosomal fragments of Streptococcus pneumoniae and used to transform a recipient strain lacking that gene, the gene was integrated at various locations in the chromosome. Such ectopic integration was demonstrated for the malM gene, and its molecular basis was analyzed with defined donor molecules consisting of ligated fragments containing the malM and sul genes of S. pneumoniae. In a recipient strain deleted in the mal region of its chromosome, these constructs gave Mal+ transformants in which the malM and sul genes were now linked, with malM located between duplicate sul segments. Ectopic integration was unstable under nonselective conditions; mal(sul) ectopic insertions were lost at a rate of 0.05% per generation. Several possible mechanisms of ectopic integration were examined. The donor molecule is most likely to be a circular form of ligated homologous and nonhomologous fragments that, after entry into the cell, undergoes circular synapsis with the recipient chromosome at the site of homology, followed by repair and additive integration.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNS K. I., THOMAS C. A., Jr ISOLATION OF HIGH MOLECULAR WEIGHT DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- Bernheimer H. P., Wermundsen I. E., Austrian R. Qualitative differences in the behavior of pneumoncoccal deoxyribonucleic acids transforming to the same capsular type. J Bacteriol. 1967 Jan;93(1):320–333. doi: 10.1128/jb.93.1.320-333.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Duncan C. H., Wilson G. A., Young F. E. Mechanism of integrating foreign DNA during transformation of Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3664–3668. doi: 10.1073/pnas.75.8.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghei O. K., Lacks S. A. Recovery of donor deoxyribonucleic acid marker activity from eclipse in pneumococcal transformation. J Bacteriol. 1967 Mar;93(3):816–829. doi: 10.1128/jb.93.3.816-829.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldenwang W. G., Banner C. D., Ollington J. F., Losick R., Hoch J. A., O'Connor M. B., Sonenshein A. L. Mapping a cloned gene under sporulation control by inserttion of a drug resistance marker into the Bacillus subtilis chromosome. J Bacteriol. 1980 Apr;142(1):90–98. doi: 10.1128/jb.142.1.90-98.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias A., Bensi G., Canosi U., Trautner T. A. Plasmid transformation in Bacillus subtilis. Alterations introduced into the recipient-homologous DNA of hybrid plasmids can be corrected in transformation. Mol Gen Genet. 1981;184(3):405–409. doi: 10.1007/BF00352513. [DOI] [PubMed] [Google Scholar]
- Lacks S. A., Dunn J. J., Greenberg B. Identification of base mismatches recognized by the heteroduplex-DNA-repair system of Streptococcus pneumoniae. Cell. 1982 Dec;31(2 Pt 1):327–336. doi: 10.1016/0092-8674(82)90126-x. [DOI] [PubMed] [Google Scholar]
- Lacks S. Integration efficiency and genetic recombination in pneumococcal transformation. Genetics. 1966 Jan;53(1):207–235. doi: 10.1093/genetics/53.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez P., Espinosa M., Lacks S. A. Physical structure and genetic expression of the sulfonamide-resistance plasmid pLS80 and its derivatives in Streptococcus pneumoniae and Bacillus subtilis. Mol Gen Genet. 1984;195(3):403–410. doi: 10.1007/BF00341440. [DOI] [PubMed] [Google Scholar]
- Lopez P., Espinosa M., Stassi D. L., Lacks S. A. Facilitation of plasmid transfer in Streptococcus pneumoniae by chromosomal homology. J Bacteriol. 1982 May;150(2):692–701. doi: 10.1128/jb.150.2.692-701.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muckerman C. C., Springhorn S. S., Greenberg B., Lacks S. A. Transformation of restriction endonuclease phenotype in Streptococcus pneumoniae. J Bacteriol. 1982 Oct;152(1):183–190. doi: 10.1128/jb.152.1.183-190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciaky D., Thomashow M. F. The sequence of the tms transcript 2 locus of the A. tumefaciens plasmid pTiA6 and characterization of the mutation in pTiA66 that is responsible for auxin attenuation. Nucleic Acids Res. 1984 Feb 10;12(3):1447–1461. doi: 10.1093/nar/12.3.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Smith M. D., Guild W. R. DNase-resistant transfer of chromosomal cat and tet insertions by filter mating in Pneumococcus. Plasmid. 1980 Jan;3(1):80–87. doi: 10.1016/s0147-619x(80)90036-0. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Smith M. D., Guild W. R. Organization and transfer of heterologous chloramphenicol and tetracycline resistance genes in pneumococcus. J Bacteriol. 1979 Aug;139(2):432–441. doi: 10.1128/jb.139.2.432-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Guild W. R. A plasmid in Streptococcus pneumoniae. J Bacteriol. 1979 Feb;137(2):735–739. doi: 10.1128/jb.137.2.735-739.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassi D. L., Lopez P., Espinosa M., Lacks S. A. Cloning of chromosomal genes in Streptococcus pneumoniae. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7028–7032. doi: 10.1073/pnas.78.11.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Knauf V. C., Nester E. W. Relationship between the limited and wide host range octopine-type Ti plasmids of Agrobacterium tumefaciens. J Bacteriol. 1981 May;146(2):484–493. doi: 10.1128/jb.146.2.484-493.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrauch Y., Lacks S. A. Nonsense mutations in the amylomaltase gene and other loci of Streptococcus pneumoniae. Mol Gen Genet. 1981;183(1):7–12. doi: 10.1007/BF00270130. [DOI] [PubMed] [Google Scholar]