Abstract
The use of novel synthetic peptides to measure peptide transport by spectrophotometric means is described. These peptides contain glycine residues alpha-substituted with thiophenol and are recognized as substrates by both peptide transport systems and intracellular peptidases of Escherichia coli (Kingsbury et al., Gilvarg, C., Proc. Natl. Acad. Sci. U.S.A. 81:4573-4576, 1984). Transport and peptidase cleavage results in the intracellular release of thiophenol, which exits rapidly from the cell. The release of thiophenol from these peptides by cell suspensions can be measured with Ellman sulfhydryl reagent [5,5'-dithiobis(2-nitrobenzoic acid)] and provides a direct determination of the rate of peptide transport. The reductions in thiophenol release from these peptides resulting from the addition of peptide competitors enable the affinities of the competitors for their transport systems to be determined. By this method, it is shown that the dipeptide transport system is more restrictive with respect to changes in the amino acid sidechains of its substrates than those of the oligopeptide transport system.
Full text
PDF
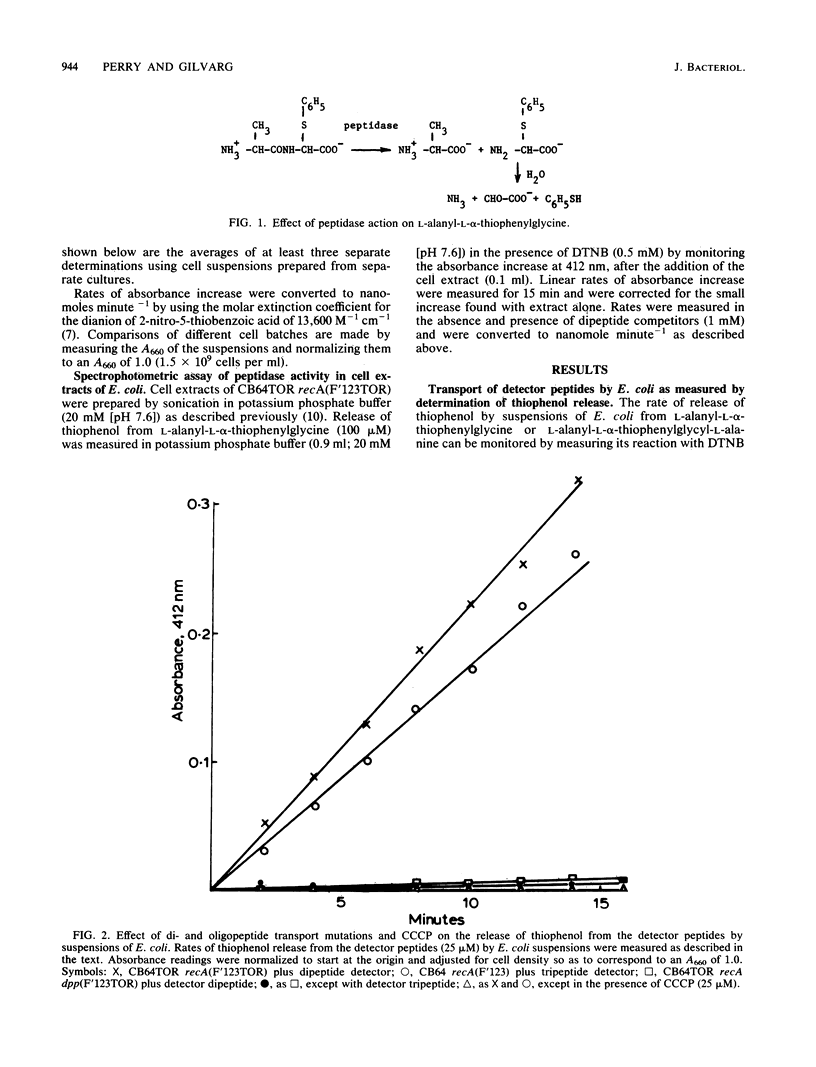
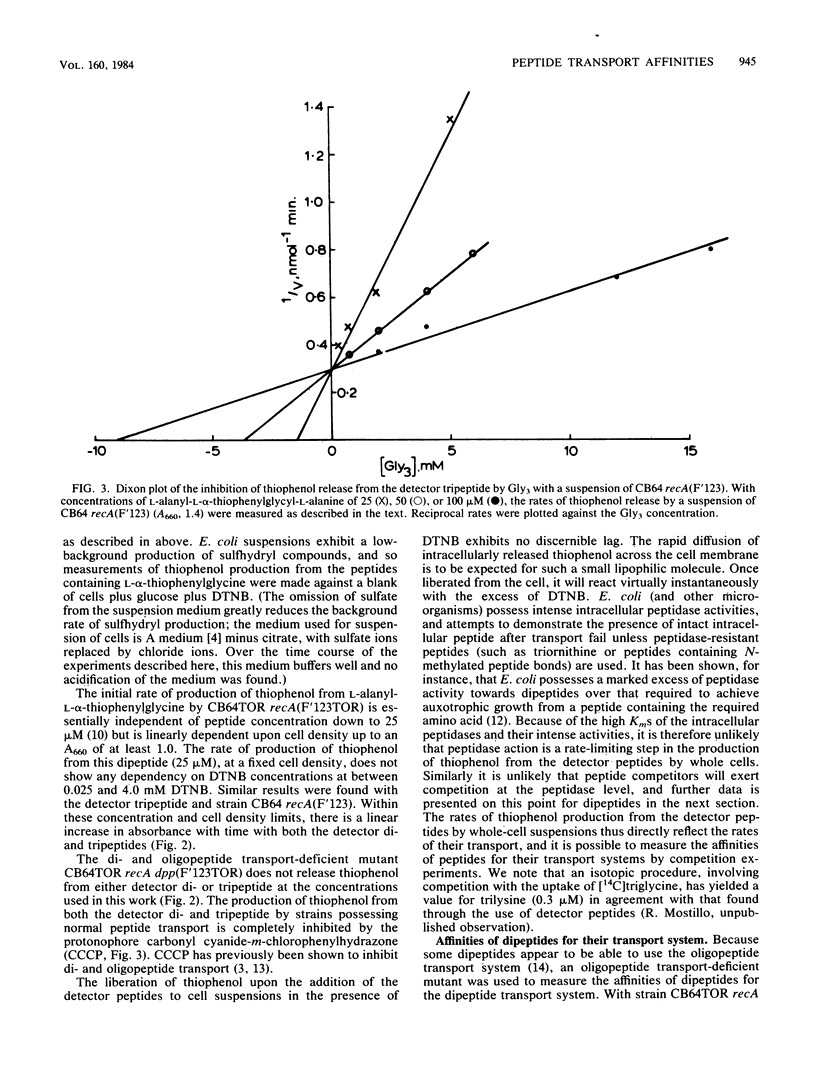



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker J. M., Naider F. Stereospecificity of tripeptide utilization in a methionine auxotroph of Escherichia coli K-12. J Bacteriol. 1974 Oct;120(1):191–196. doi: 10.1128/jb.120.1.191-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm J. C., Kingsbury W. D., Perry D., Gilvarg C. The use of cysteinyl peptides to effect portage transport of sulfhydryl-containing compounds in Escherichia coli. J Biol Chem. 1983 Dec 25;258(24):14850–14855. [PubMed] [Google Scholar]
- Cowell J. L. Energetics of glycylglycine transport in Escherichia coli. J Bacteriol. 1974 Oct;120(1):139–146. doi: 10.1128/jb.120.1.139-146.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M., Guardiola J., Lamberti A., Iaccarino M. Escherichia coli K-12 mutants altered in the transport systems for oligo- and dipeptides. J Bacteriol. 1973 Nov;116(2):751–756. doi: 10.1128/jb.116.2.751-756.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- KESSEL D., LUBIN M. On the distinction between peptidase activity and peptide transport. Biochim Biophys Acta. 1963 Jun 4;71:656–663. doi: 10.1016/0006-3002(63)91139-9. [DOI] [PubMed] [Google Scholar]
- Kingsbury W. D., Boehm J. C., Perry D., Gilvarg C. Portage of various compounds into bacteria by attachment to glycine residues in peptides. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4573–4576. doi: 10.1073/pnas.81.14.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J. W., Bell G. Direct determination of the properties of peptide transport systems in Escherichia coli, using a fluorescent-labeling procedure. J Bacteriol. 1979 Jan;137(1):447–455. doi: 10.1128/jb.137.1.447-455.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J. W., Gilvarg C. The role of the terminal carboxyl group on peptide transport in Escherichia coli. J Biol Chem. 1968 Jan 25;243(2):335–340. [PubMed] [Google Scholar]
- Payne J. W., Nisbet T. M. Limitations to the use of radioactively labelled substrates for studying peptide transport in microorganisms. FEBS Lett. 1980 Sep 22;119(1):73–76. doi: 10.1016/0014-5793(80)81000-3. [DOI] [PubMed] [Google Scholar]
- Payne J. W. Oligopeptide transport in Escherichia coli. Specificity with respect to side chain and distinction from dipeptide transport. J Biol Chem. 1968 Jun 25;243(12):3395–3403. [PubMed] [Google Scholar]
- Payne J. W. Variations in the peptidase activities of Escherichia coli in response to environmental changes. J Gen Microbiol. 1972 Jul;71(2):281–291. doi: 10.1099/00221287-71-2-281. [DOI] [PubMed] [Google Scholar]
- Perry D., Gilvarg C. Metabolism of alanylalanyl-S-[N-(2-thioethyl)]aminopyridine-2, 6-dicarboxylic acid]cysteine by suspensions of Escherichia coli. J Biol Chem. 1983 Dec 25;258(24):14856–14860. [PubMed] [Google Scholar]
- Staros J. V., Knowles J. R. Photoaffinity inhibition of dipeptide transport in Escherichia coli. Biochemistry. 1978 Aug 8;17(16):3321–3325. doi: 10.1021/bi00609a023. [DOI] [PubMed] [Google Scholar]



