Abstract
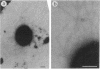
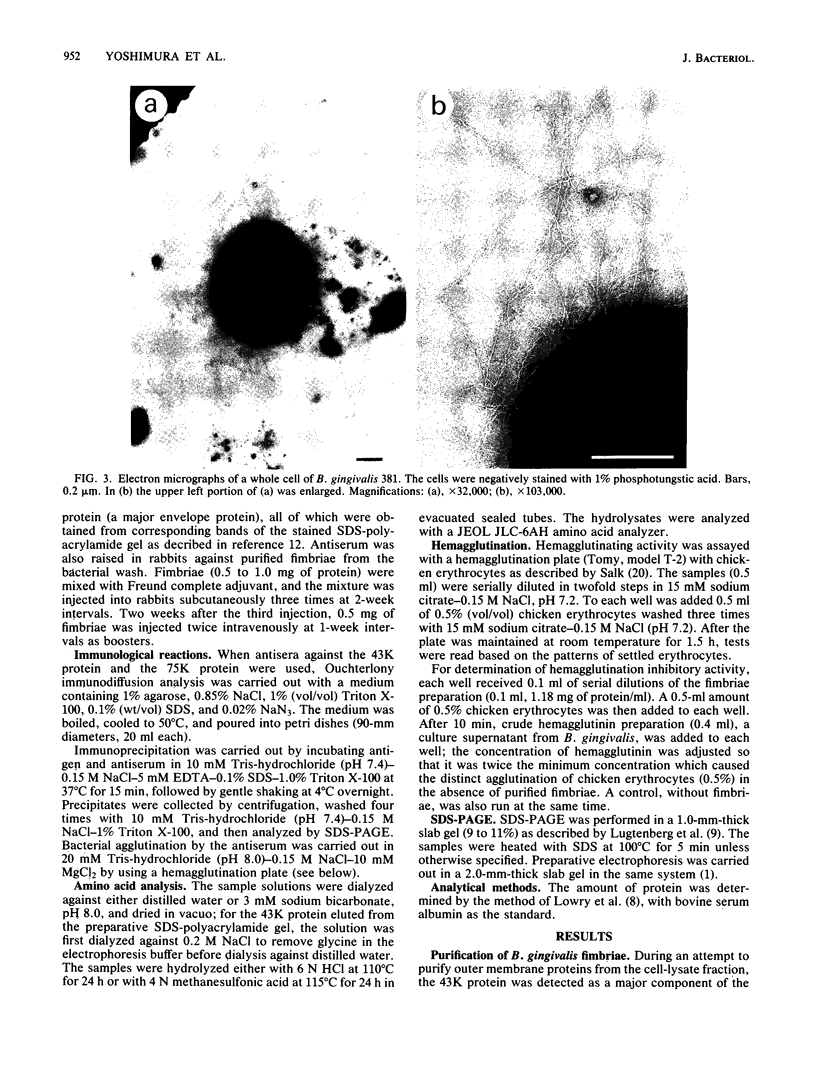
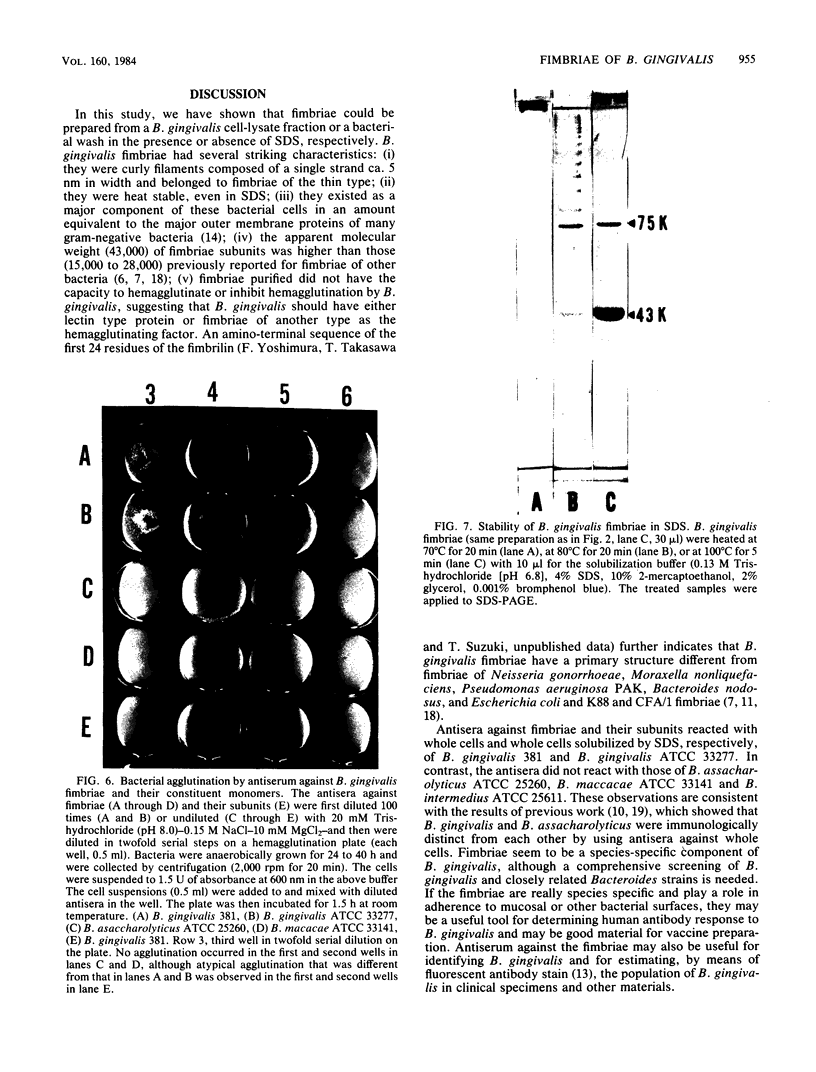
Fimbriae and their constituent protein (fimbrilin) were purified to homogeneity from the bacterial wash fluid and cell lysate fraction, respectively, of Bacteroides gingivalis 381. Fimbriae, observed by negative staining, were curly, single-stranded filaments with a diameter of ca. 5 nm. The apparent molecular weight of the fimbrilin was 43,000. Fimbriae were resistant to sodium dodecyl sulfate denaturation at 70 degrees C. Heating at 100 degrees C in sodium dodecyl sulfate was needed to completely dissociate them to monomers of fimbrilin. Different sets of antigenic determinants seemed to be exposed on the surfaces of fimbriae and sodium dodecyl sulfate-denatured fimbrilin. Purified fimbriae did not show either hemagglutinating activity or hemagglutination inhibitory activity, although it has been inferred on the basis of circumstantial evidence that fimbriae are correlated to hemagglutinating activity of the organism. Hemagglutinin activity, however, was detected in culture supernatant, and this observation suggests that fimbriae of a different type or a lectin-like protein may be acting as hemagglutinin in B. gingivalis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deal C. D., Kaplan S. Solubilization, isolation, and immunochemical characterization of the major outer membrane protein from Rhodopseudomonas sphaeroides. J Biol Chem. 1983 May 25;258(10):6524–6529. [PubMed] [Google Scholar]
- Hofstra H., Dankert J. Porin from the outer membrane of Escherichia coli: immunological characterization of native and heat-dissociated forms. J Gen Microbiol. 1981 Aug;125(2):285–292. doi: 10.1099/00221287-125-2-285. [DOI] [PubMed] [Google Scholar]
- Klemm P. Primary structure of the CFA1 fimbrial protein from human enterotoxigenic Escherichia coli strains. Eur J Biochem. 1982 May 17;124(2):339–348. doi: 10.1111/j.1432-1033.1982.tb06597.x. [DOI] [PubMed] [Google Scholar]
- Klemm P. The complete amino-acid sequence of the K88 antigen, a fimbrial protein from Escherichia coli. Eur J Biochem. 1981 Jul;117(3):617–627. doi: 10.1111/j.1432-1033.1981.tb06382.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Mansheim B. J., Coleman S. E. Immunochemical differences between oral and nonoral strains of Bacteroides asaccharolyticus. Infect Immun. 1980 Feb;27(2):589–596. doi: 10.1128/iai.27.2.589-596.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKern N. M., O'Donnell I. J., Inglis A. S., Stewart D. J., Clark B. L. Amino acid sequence of pilin from Bacteroides nodosus (strain 198), the causative organism of ovine footrot. FEBS Lett. 1983 Nov 28;164(1):149–153. doi: 10.1016/0014-5793(83)80039-8. [DOI] [PubMed] [Google Scholar]
- Mihara K., Blobel G. The four cytoplasmically made subunits of yeast mitochondrial cytochrome c oxidase are synthesized individually and not as a polyprotein. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4160–4164. doi: 10.1073/pnas.77.7.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton C., Hammond P. G., Slots J., Reed M. J., Genco R. J. Identification of Bacteroides gingivalis by fluorescent antibody staining. Ann Microbiol (Paris) 1981 Jul-Aug;132B(1):69–83. [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Okuda K., Takazoe I. Haemagglutinating activity of Bacteroides melaninogenicus. Arch Oral Biol. 1974 May;19(5):415–416. doi: 10.1016/0003-9969(74)90184-8. [DOI] [PubMed] [Google Scholar]
- Ottow J. C. Ecology, physiology, and genetics of fimbriae and pili. Annu Rev Microbiol. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- Reed M. J., Slots J., Mouton C., Genco R. J. Antigenic studies of oral and nonoral black-pigmented Bacteroides strains. Infect Immun. 1980 Aug;29(2):564–574. doi: 10.1128/iai.29.2.564-574.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. I., Emerson S. U., Shaper J. H., Bernard P. D., Glazer A. N. Classification of Bacillus subtilis flagellins. J Bacteriol. 1977 Apr;130(1):200–204. doi: 10.1128/jb.130.1.200-204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Direct hemagglutination technique for differentiating Bacteroides asaccharolyticus oral strains from nonoral strains. J Clin Microbiol. 1979 Sep;10(3):371–373. doi: 10.1128/jcm.10.3.371-373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Woo D. D., Holt S. C., Leadbetter E. R. Ultrastructure of Bacteroides species: Bacteroides asaccharolyticus, Bacteroides fragilis, Bacteroides melaninogenicus subspecies melaninogenicus, and B. melaninogenicus subspecies intermedius. J Infect Dis. 1979 May;139(5):534–546. doi: 10.1093/infdis/139.5.534. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nishikata M., Suzuki T., Hoover C. I., Newbrun E. Characterization of a trypsin-like protease from the bacterium Bacteroides gingivalis isolated from human dental plaque. Arch Oral Biol. 1984;29(7):559–564. doi: 10.1016/0003-9969(84)90078-5. [DOI] [PubMed] [Google Scholar]