Abstract
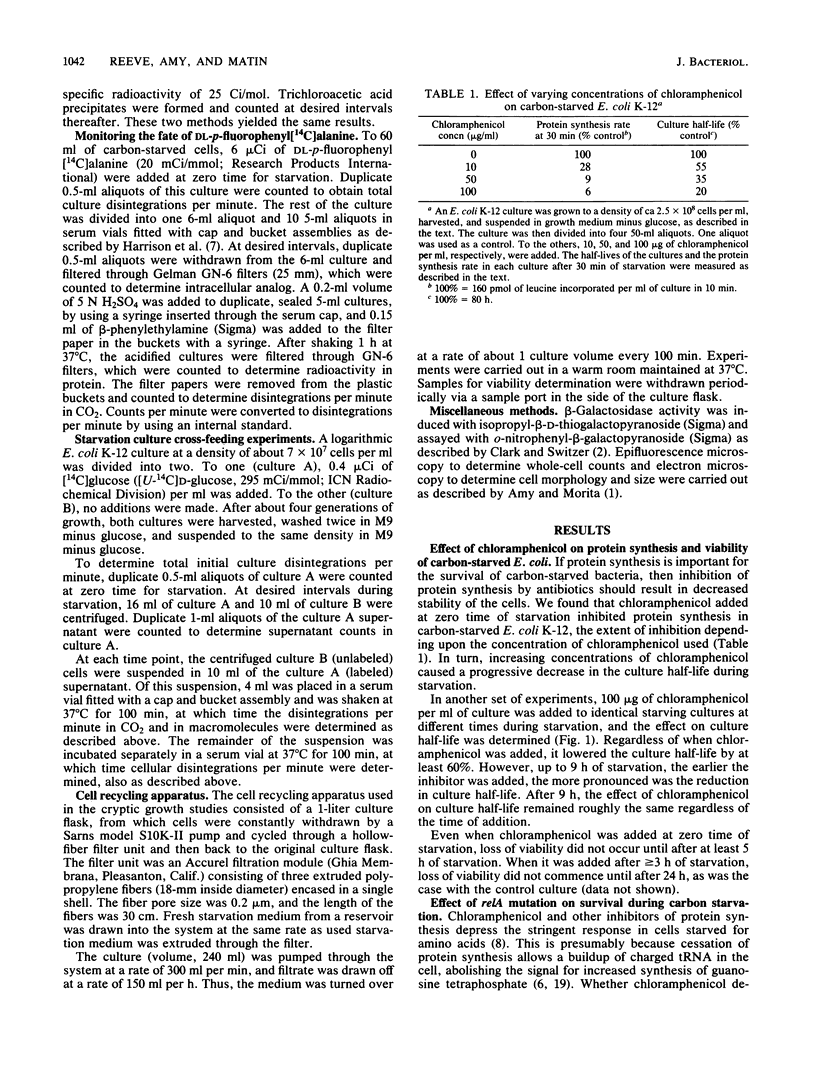
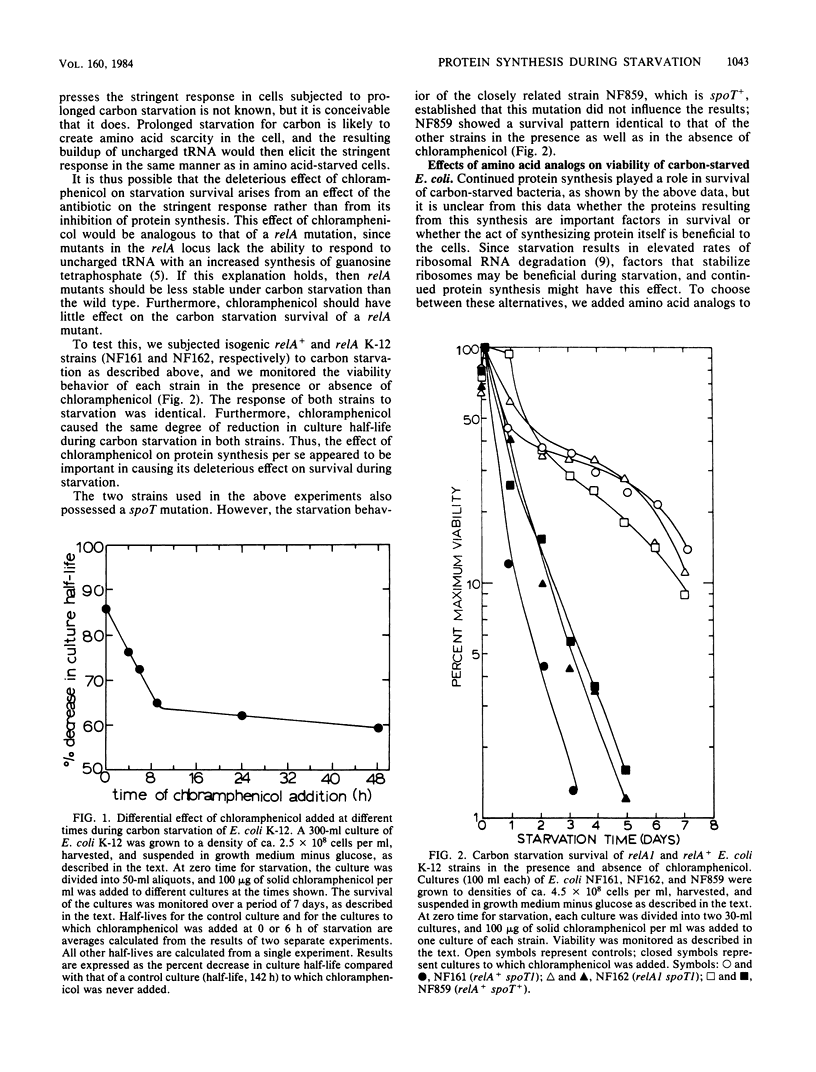
In a typical Escherichia coli K-12 culture starved for glucose, 50% of the cells lose viability in ca. 6 days (Reeve et al., J. Bacteriol. 157:758-763, 1984). Inhibition of protein synthesis by chloramphenicol resulted in a more rapid loss of viability in glucose-starved E. coli K-12 cultures. The more chloramphenicol added (i.e., the more protein synthesis was inhibited) and the earlier during starvation it was added, the greater was its effect on culture viability. Chloramphenicol was found to have the same effect on a relA strain as on an isogenic relA+ strain of E. coli. Addition of the amino acid analogs S-2-aminoethylcysteine, 7-azatryptophan, and p-fluorophenylalanine to carbon-starved cultures to induce synthesis of abnormal proteins had an effect on viability similar to that observed when 50 micrograms of chloramphenicol per ml was added at zero time for starvation. Both chloramphenicol and the amino acid analogs had delayed effects on viability, compared with their effects on synthesis of normal proteins. The need for protein synthesis did not arise from cryptic growth, since no cryptic growth of the starving cells was observed under the conditions used. From these and previous results obtained from work with peptidase-deficient mutants of E. coli K-12 and Salmonella typhimurium LT2 (Reeve et al., J. Bacteriol. 157:758-763, 1984), we concluded that a number of survival-related proteins are synthesized by E. coli K-12 cells as a response to carbon starvation. These proteins are largely synthesized during the early hours of starvation, but their continued activity is required for long-term survival.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy P. S., Morita R. Y. Starvation-survival patterns of sixteen freshly isolated open-ocean bacteria. Appl Environ Microbiol. 1983 Mar;45(3):1109–1115. doi: 10.1128/aem.45.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiil N. P., Willumsen B. M., Friesen J. D., von Meyenburg K. Interaction of alleles of the relA, relC and spoT genes in Escherichia coli: analysis of the interconversion of GTP, ppGpp and pppGpp. Mol Gen Genet. 1977 Jan 7;150(1):87–101. doi: 10.1007/BF02425329. [DOI] [PubMed] [Google Scholar]
- Fowden L., Lewis D., Tristram H. Toxic amino acids: their action as antimetabolites. Adv Enzymol Relat Areas Mol Biol. 1967;29:89–163. doi: 10.1002/9780470122747.ch3. [DOI] [PubMed] [Google Scholar]
- Gallant J. A. Stringent control in E. coli. Annu Rev Genet. 1979;13:393–415. doi: 10.1146/annurev.ge.13.120179.002141. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. A role of aminoacyl-tRNA in the regulation of protein breakdown in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Feb;68(2):362–366. doi: 10.1073/pnas.68.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M. J., Wright R. T., Morita R. Y. Method for measuring mineralization in lake sediments. Appl Microbiol. 1971 Apr;21(4):698–702. doi: 10.1128/am.21.4.698-702.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Kjeldgaard N. O. Metabolism of guanosine tetraphosphate in Escherichia coli. Eur J Biochem. 1972 Jul 24;28(3):316–326. doi: 10.1111/j.1432-1033.1972.tb01916.x. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. The intracellular turnover of protein and nucleic acids and its role in biochemical differentiation. Bacteriol Rev. 1960 Sep;24(3):289–308. doi: 10.1128/br.24.3.289-308.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Grootjans A., Hogenhuis H. Influence of dilution rate on enzymes of intermediary metabolism in two freshwater bacteria grown in continuous culture. J Gen Microbiol. 1976 Jun;94(2):323–332. doi: 10.1099/00221287-94-2-323. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- Pao C. C., Gallant J. A gene involved in the metabolic control of ppGpp synthesis. Mol Gen Genet. 1978 Jan 17;158(3):271–277. doi: 10.1007/BF00267198. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. The effect of amino acid analogues on growth and protein synthesis in microorganisms. Bacteriol Rev. 1962 Dec;26:398–420. doi: 10.1128/br.26.4.398-420.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYAN F. J. Bacterial mutation in a stationary phase and the question of cell turnover. J Gen Microbiol. 1959 Dec;21:530–549. doi: 10.1099/00221287-21-3-530. [DOI] [PubMed] [Google Scholar]
- Reeve C. A., Bockman A. T., Matin A. Role of protein degradation in the survival of carbon-starved Escherichia coli and Salmonella typhimurium. J Bacteriol. 1984 Mar;157(3):758–763. doi: 10.1128/jb.157.3.758-763.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John A. C., Conklin K., Rosenthal E., Goldberg A. L. Further evidence for the involvement of charged tRNA and guanosine tetraphosphate in the control of protein degradation in Escherichia coli. J Biol Chem. 1978 Jun 10;253(11):3945–3951. [PubMed] [Google Scholar]
- Willetts N. S. Intracellular protein breakdown in growing cells of Escherichia coli. Biochem J. 1967 May;103(2):462–466. doi: 10.1042/bj1030462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen C., Green L., Miller C. G. Degradation of intracellular protein in Salmonella typhimurium peptidase mutants. J Mol Biol. 1980 Oct 15;143(1):21–33. doi: 10.1016/0022-2836(80)90122-9. [DOI] [PubMed] [Google Scholar]
- Zychlinsky E., Matin A. Effect of starvation on cytoplasmic pH, proton motive force, and viability of an acidophilic bacterium, Thiobacillus acidophilus. J Bacteriol. 1983 Jan;153(1):371–374. doi: 10.1128/jb.153.1.371-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



