Abstract
The function of the epsilon subunit of the Escherichia coli proton-translocating ATPase has been examined by using a mutant defective in the uncC gene. Strains with a defective uncC gene show a reduction in both growth yield and growth rate that is more severe than for other unc mutants; this deleterious effect is shown to be a result of the ATPase activity of the F1 complex which is missing the epsilon subunit. In addition, the epsilon-deficient F1 is bound less tightly to the membrane. These data suggest that, in vivo, the epsilon subunit is capable of inhibiting the ATPase activity of F1 and also functions in the binding of F1 to F0.
Full text
PDF
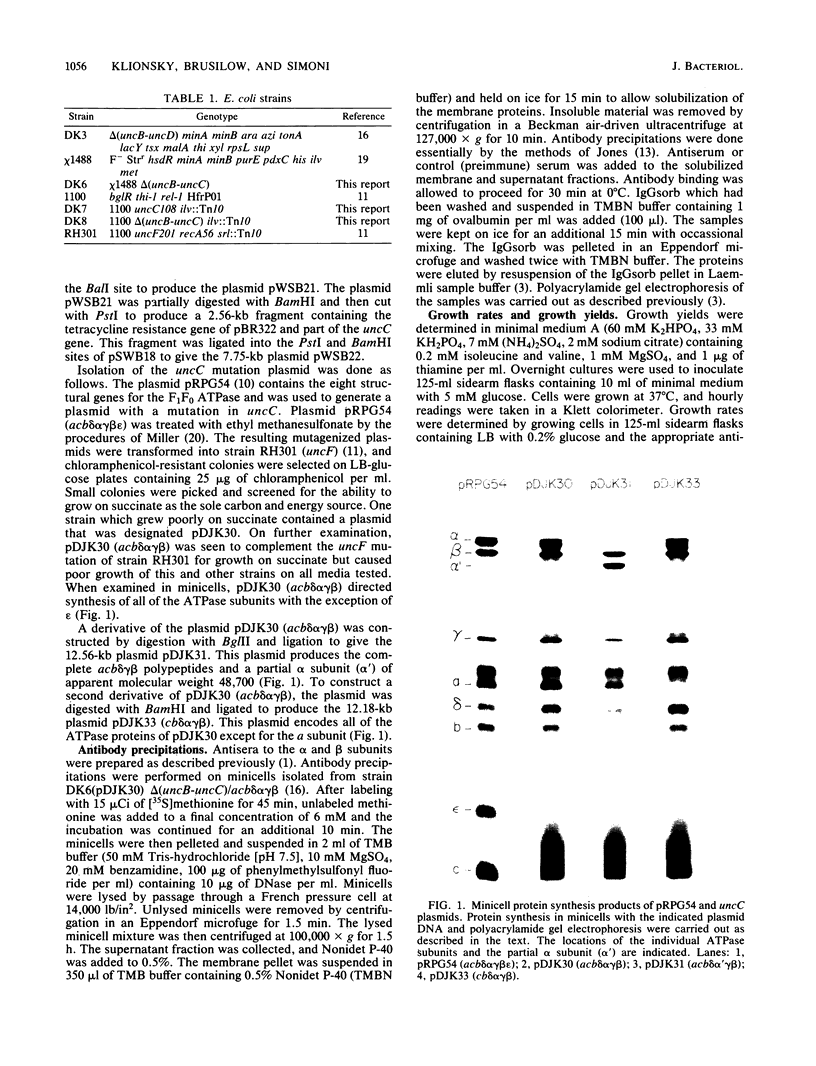
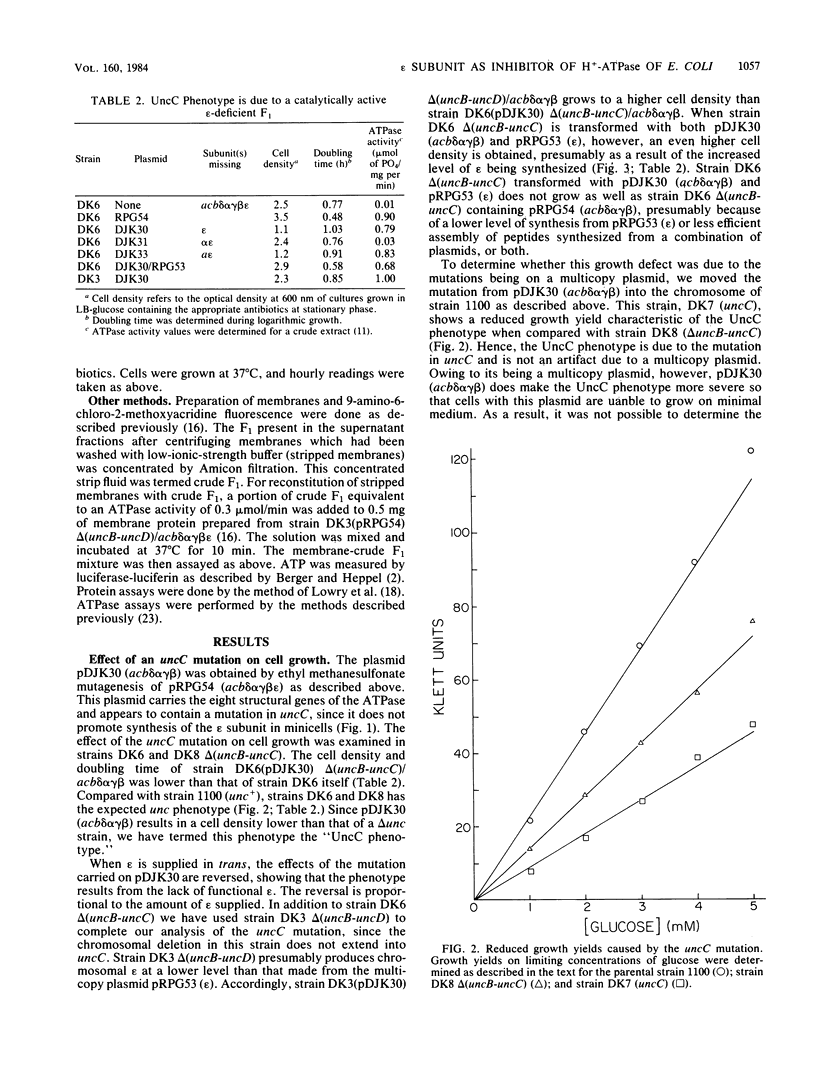
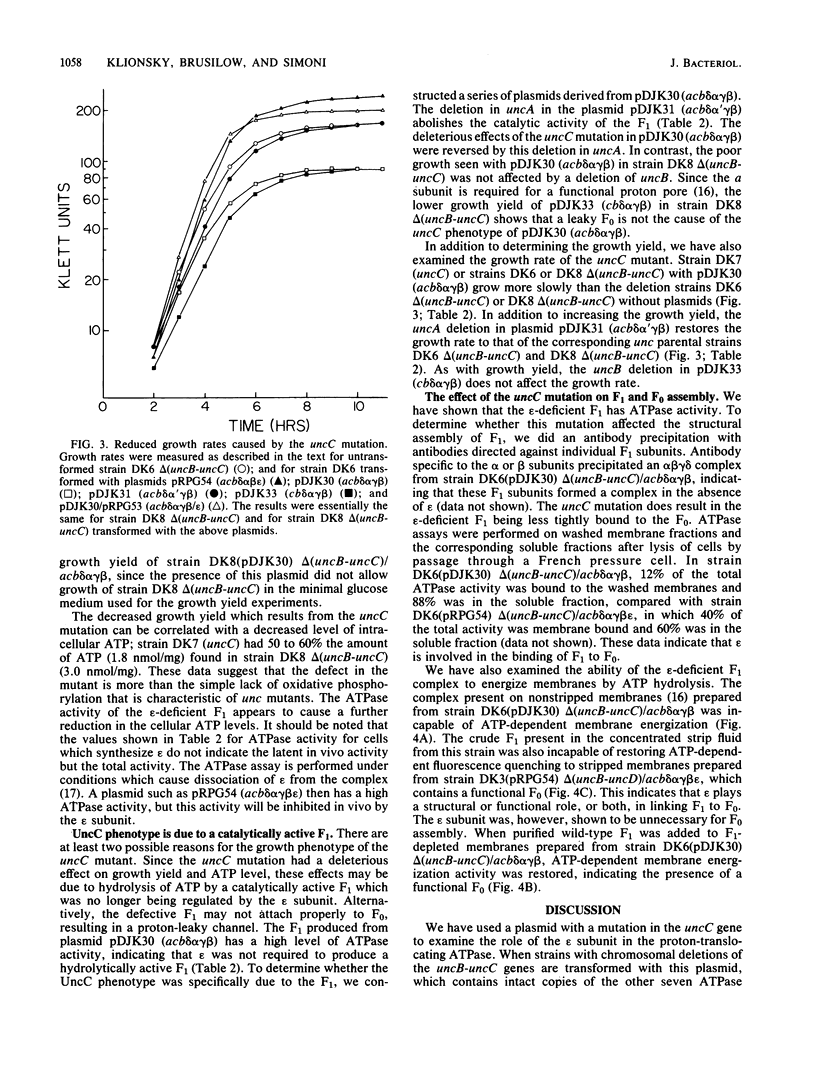
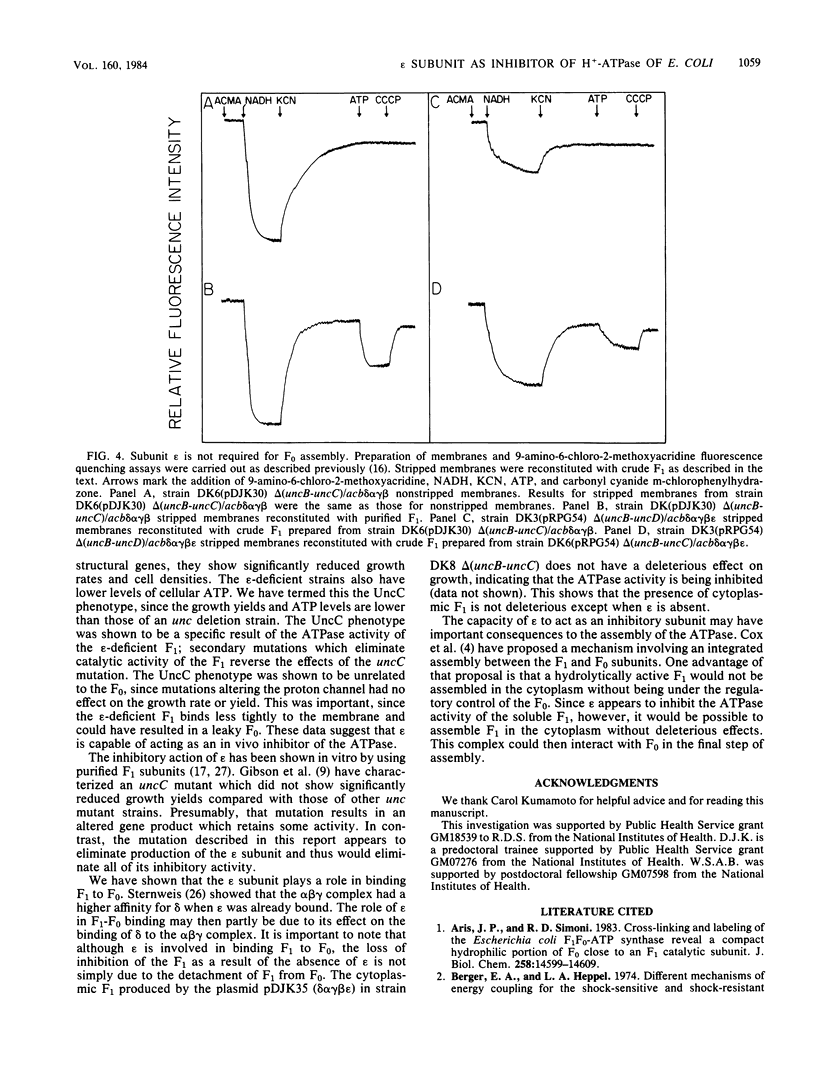

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aris J. P., Simoni R. D. Cross-linking and labeling of the Escherichia coli F1F0-ATP synthase reveal a compact hydrophilic portion of F0 close to an F1 catalytic subunit. J Biol Chem. 1983 Dec 10;258(23):14599–14609. [PubMed] [Google Scholar]
- Brusilow W. S., Gunsalus R. P., Hardeman E. C., Decker K. P., Simoni R. D. In vitro synthesis of the F0 and F1 components of the proton translocating ATPase of Escherichia coli. J Biol Chem. 1981 Apr 10;256(7):3141–3144. [PubMed] [Google Scholar]
- Cox G. B., Downie J. A., Langman L., Senior A. E., Ash G., Fayle D. R., Gibson F. Assembly of the adenosine triphosphatase complex in Escherichia coli: assembly of F0 is dependent on the formation of specific F1 subunits. J Bacteriol. 1981 Oct;148(1):30–42. doi: 10.1128/jb.148.1.30-42.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S. D. ATP causes a large change in the conformation of the isolated alpha subunit of Escherichia coli F1 ATPase. J Biol Chem. 1980 Dec 25;255(24):11857–11860. [PubMed] [Google Scholar]
- Futai M. Reconstitution of ATPase activity from the isolated alpha, beta, and gamma subunits of the coupling factor, F1, of Escherichia coli. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1231–1237. doi: 10.1016/0006-291x(77)91138-x. [DOI] [PubMed] [Google Scholar]
- Futai M., Sternweis P. C., Heppel L. A. Purification and properties of reconstitutively active and inactive adenosinetriphosphatase from Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2725–2729. doi: 10.1073/pnas.71.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Cox G. B., Downie J. A., Radik J. A mutation affecting a second component of the F0 portion of the magnesium ion-stimulated adenosine triphosphatase of Escherichia coli K12. The uncC424 allele. Biochem J. 1977 Apr 15;164(1):193–198. doi: 10.1042/bj1640193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Brusilow W. S., Simoni R. D. Gene order and gene-polypeptide relationships of the proton-translocating ATPase operon (unc) of Escherichia coli. Proc Natl Acad Sci U S A. 1982 Jan;79(2):320–324. doi: 10.1073/pnas.79.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert R., Brusilow W. S., Gunsalus R. P., Klionsky D. J., Simoni R. D. Escherichia coli mutants defective in the uncH gene. J Bacteriol. 1983 Jan;153(1):416–422. doi: 10.1128/jb.153.1.416-422.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Miki T., Tamura F., Yura T., Futai M. Specialized transducing phage lambda carrying the genes for coupling factor of oxidative phosphorylation of Escherichia coli: increased synthesis of coupling factor on induction of prophage lambda asn. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1126–1130. doi: 10.1073/pnas.76.3.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa H., Noumi T., Futai M., Nitta T. Escherichia coli mutants defective in the gamma subunit of proton-translocating ATPase: intracistronic mapping of the defective site and the biochemical properties of the mutants. Arch Biochem Biophys. 1983 Jun;223(2):521–532. doi: 10.1016/0003-9861(83)90617-3. [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Brusilow W. S., Simoni R. D. Assembly of a functional F0 of the proton-translocating ATPase of Escherichia coli. J Biol Chem. 1983 Aug 25;258(16):10136–10143. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laget P. P., Smith J. B. Inhibitory properties of endogenous subunit epsilon in the Escherichia coli F1 ATPase. Arch Biochem Biophys. 1979 Oct 1;197(1):83–89. doi: 10.1016/0003-9861(79)90222-4. [DOI] [PubMed] [Google Scholar]
- Lötscher H. R., deJong C., Capaldi R. A. Interconversion of high and low adenosinetriphosphatase activity forms of Escherichia coli F1 by the detergent lauryldimethylamine oxide. Biochemistry. 1984 Aug 28;23(18):4140–4143. doi: 10.1021/bi00313a020. [DOI] [PubMed] [Google Scholar]
- Meagher R. B., Tait R. C., Betlach M., Boyer H. W. Protein expression in E. coli minicells by recombinant plasmids. Cell. 1977 Mar;10(3):521–536. doi: 10.1016/0092-8674(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Richter M. L., Patrie W. J., McCarty R. E. Preparation of the epsilon subunit and epsilon subunit-deficient chloroplast coupling factor 1 in reconstitutively active forms. J Biol Chem. 1984 Jun 25;259(12):7371–7373. [PubMed] [Google Scholar]
- Satre M., Lunardi J., Pougeois R., Vignais P. V. Inactivation of Escherichia coli BF1-ATPase by dicyclohexylcarbodiimide. Chemical modification of the beta subunit. Biochemistry. 1979 Jul 10;18(14):3134–3140. doi: 10.1021/bi00581a034. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Shandell A. Energy transduction in Escherichia coli. Genetic alteration of a membrane polypeptide of the (Ca2+,Mg2+)-ATPase. J Biol Chem. 1975 Dec 25;250(24):9421–9427. [PubMed] [Google Scholar]
- Smith J. B., Sternweis P. C. Purification of membrane attachment and inhibitory subunits of the proton translocating adenosine triphosphatase from Escherichia coli. Biochemistry. 1977 Jan 25;16(2):306–311. doi: 10.1021/bi00621a023. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Sternweis P. C. Restoration of coupling factor activity to Escherichia coli ATPase missing the delta subunit. Biochem Biophys Res Commun. 1975 Feb 3;62(3):764–771. doi: 10.1016/0006-291x(75)90465-9. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Smith J. B. Characterization of the inhibitory (epsilon) subunit of the proton-translocating adenosine triphosphatase from Escherichia coli. Biochemistry. 1980 Feb 5;19(3):526–531. doi: 10.1021/bi00544a021. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C. The epsilon subunit of Escherichia coli coupling factor 1 is required for its binding to the cytoplasmic membrane. J Biol Chem. 1978 May 10;253(9):3123–3128. [PubMed] [Google Scholar]




