Abstract
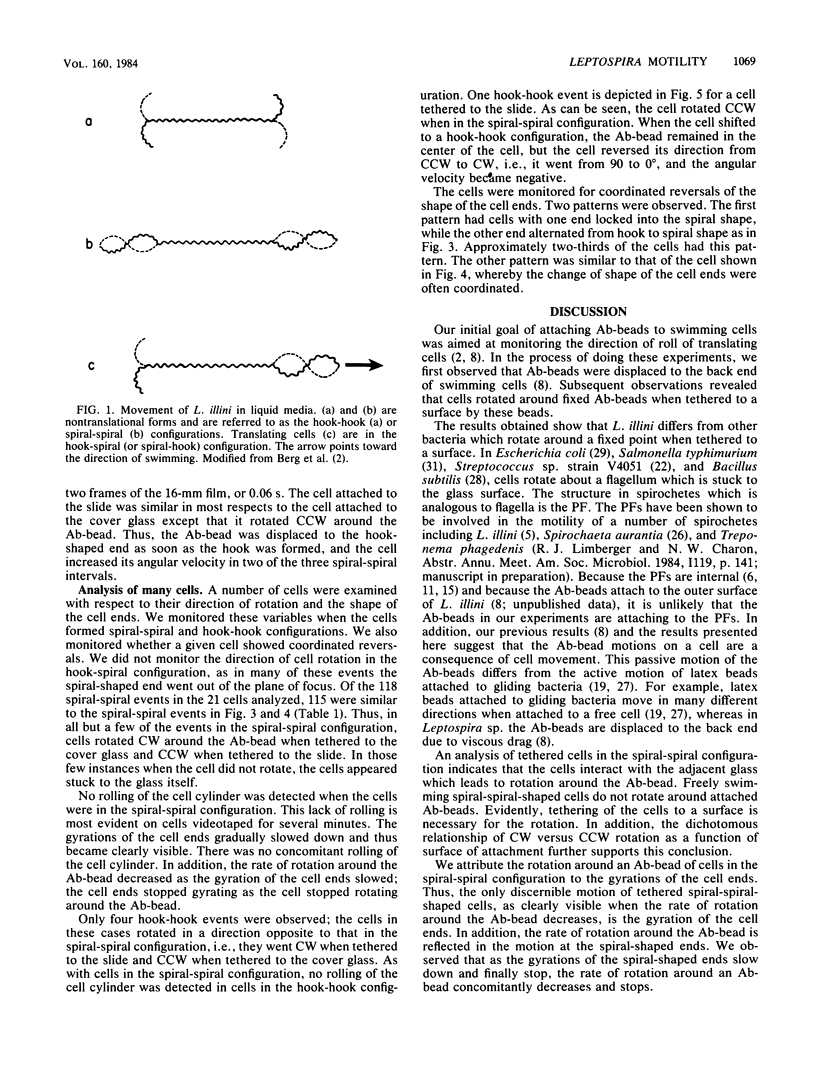
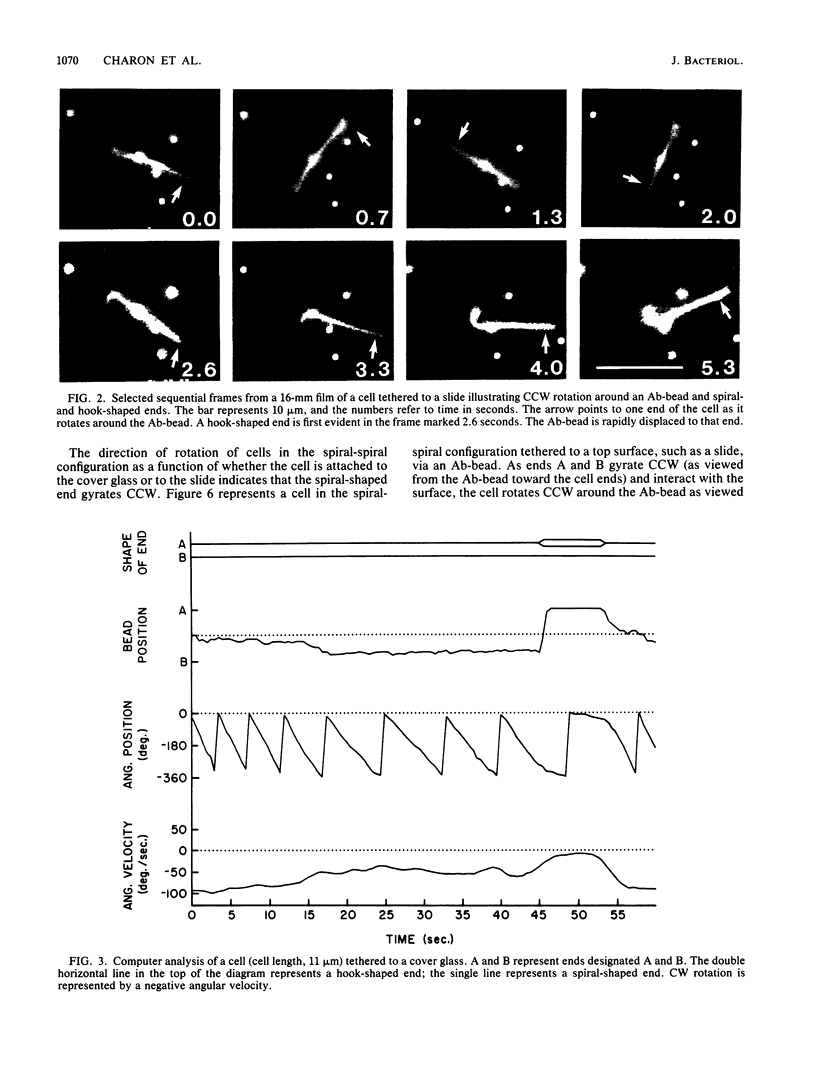
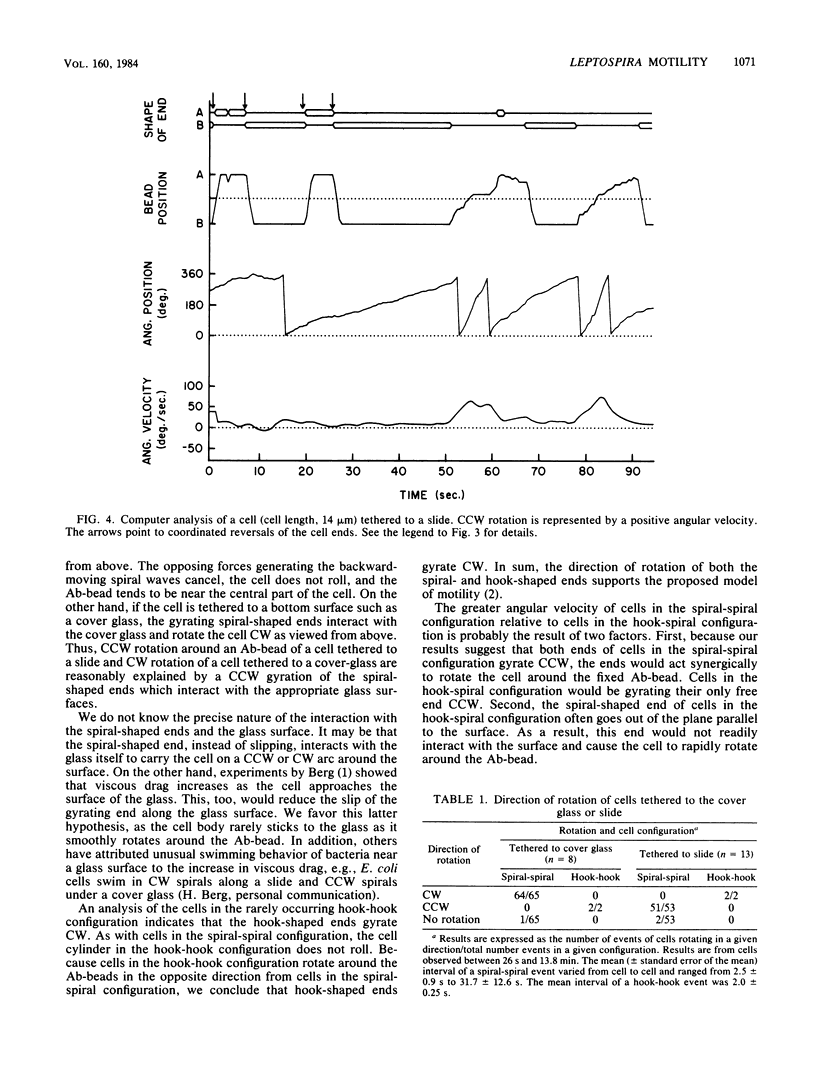
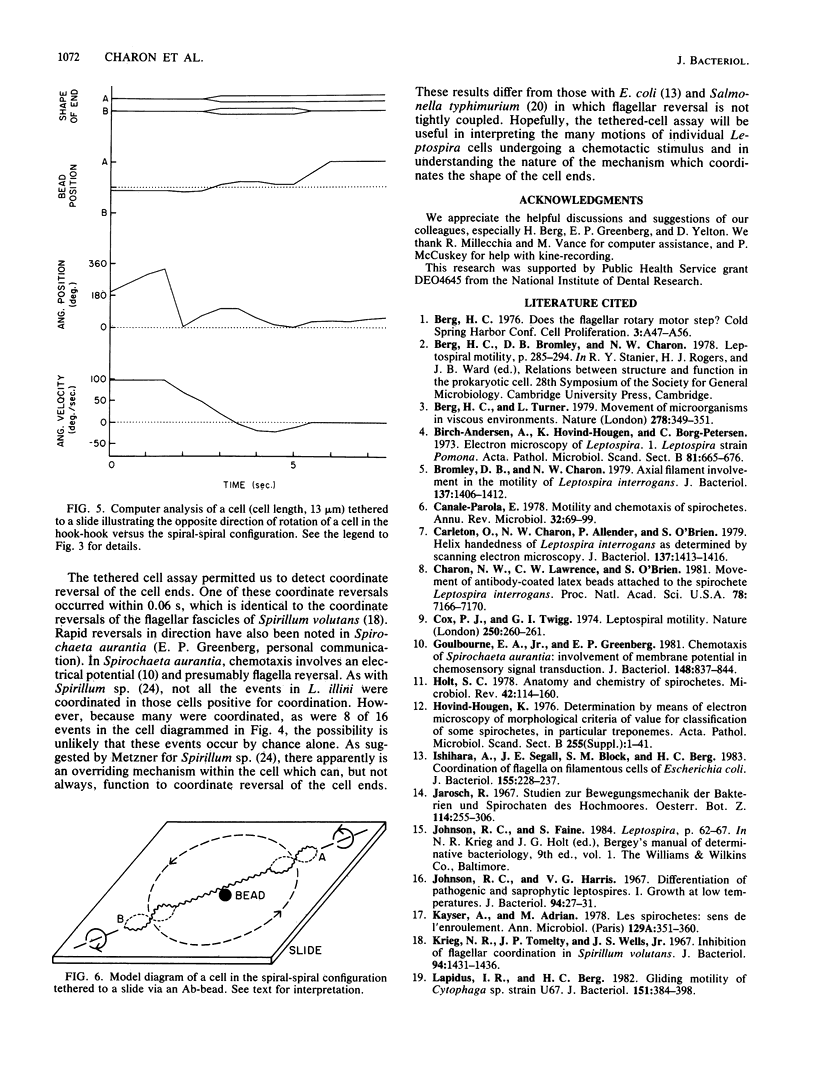
A model of Leptospira motility was recently proposed. One element of the model states that in translating cells the anterior spiral-shaped end gyrates counterclockwise and the posterior hook-shaped end gyrates clockwise. We tested these predictions by analyzing cells tethered to a glass surface. Leptospira illini was incubated with antibody-coated latex beads (Ab-beads). These beads adhered to the cells, and subsequently some cells became attached to either the slide or the cover glass via the Ab-beads. As previously reported, these cells rapidly moved back and forth across the surface of the beads. In addition, a general trend was observed: cells tethered to the cover glass rotated clockwise around the Ab-bead; cells tethered to the slide rotated counterclockwise around the Ab-bead. A computer-aided microcinematographic analysis of tethered cells indicated that the direction of rotation of cells around the Ab-bead was a function of both the surface of attachment and the shape of the cell ends. The results can best be explained by assuming that the gyrating ends interact with the glass surface to cause rotation around the Ab-beads. The analysis obtained indicates that the hook- and spiral-shaped ends rotate in the directions predicted by the model. In addition, the tethered cell assay permitted detection of rapid, coordinated reversals of the cell ends, e.g., cells rapidly switched from a hook-spiral configuration to a spiral-hook configuration. These results suggest the existance of a mechanism which coordinates the shape of the cell ends of L. illini.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg H. C., Turner L. Movement of microorganisms in viscous environments. Nature. 1979 Mar 22;278(5702):349–351. doi: 10.1038/278349a0. [DOI] [PubMed] [Google Scholar]
- Birch-Andersen A., Hovind Hougen K., Borg-Petersen C. Electron microscopy of Leptospira. 1. Leptospira strain Pomona. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Dec;81(6):665–676. doi: 10.1111/j.1699-0463.1973.tb02258.x. [DOI] [PubMed] [Google Scholar]
- Bromley D. B., Charon N. W. Axial filament involvement in the motility of Leptospira interrogans. J Bacteriol. 1979 Mar;137(3):1406–1412. doi: 10.1128/jb.137.3.1406-1412.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canale-Parola E. Motility and chemotaxis of spirochetes. Annu Rev Microbiol. 1978;32:69–99. doi: 10.1146/annurev.mi.32.100178.000441. [DOI] [PubMed] [Google Scholar]
- Carleton O., Charon N. W., Allender P., O'Brien S. Helix handedness of Leptospira interrogans as determined by scanning electron microscopy. J Bacteriol. 1979 Mar;137(3):1413–1416. doi: 10.1128/jb.137.3.1413-1416.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon N. W., Lawrence C. W., O'Brien S. Movement of antibody-coated latex beads attached to the spirochete Leptospira interrogans. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7166–7170. doi: 10.1073/pnas.78.11.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox P. J., Twigg G. I. Leptospiral motility. Nature. 1974 Jul 19;250(463):260–261. doi: 10.1038/250260a0. [DOI] [PubMed] [Google Scholar]
- Goulbourne E. A., Jr, Greenberg E. P. Chemotaxis of Spirochaeta aurantia: involvement of membrane potential in chemosensory signal transduction. J Bacteriol. 1981 Dec;148(3):837–844. doi: 10.1128/jb.148.3.837-844.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C. Anatomy and chemistry of spirochetes. Microbiol Rev. 1978 Mar;42(1):114–160. doi: 10.1128/mr.42.1.114-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovind-Hougen K. Determination by means of electron microscopy of morphological criteria of value for classification of some spirochetes, in particular treponemes. Acta Pathol Microbiol Scand Suppl. 1976;(255):1–41. [PubMed] [Google Scholar]
- Ishihara A., Segall J. E., Block S. M., Berg H. C. Coordination of flagella on filamentous cells of Escherichia coli. J Bacteriol. 1983 Jul;155(1):228–237. doi: 10.1128/jb.155.1.228-237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Harris V. G. Differentiation of pathogenic and saprophytic letospires. I. Growth at low temperatures. J Bacteriol. 1967 Jul;94(1):27–31. doi: 10.1128/jb.94.1.27-31.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser A., Adrian M. Les spirochètes: sens de l'enroulement. Ann Microbiol (Paris) 1978 Apr;129(3):351–360. [PubMed] [Google Scholar]
- Krieg N. R., Tomelty J. P., Wells J. S., Jr Inhibitio of flagellar coordination in Spirillum volutans. J Bacteriol. 1967 Nov;94(5):1431–1436. doi: 10.1128/jb.94.5.1431-1436.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus I. R., Berg H. C. Gliding motility of Cytophaga sp. strain U67. J Bacteriol. 1982 Jul;151(1):384–398. doi: 10.1128/jb.151.1.384-398.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Han D. P. Asynchronous switching of flagellar motors on a single bacterial cell. Cell. 1983 Jan;32(1):109–117. doi: 10.1016/0092-8674(83)90501-9. [DOI] [PubMed] [Google Scholar]
- Macnab R., Koshland D. E., Jr Bacterial motility and chemotaxis: light-induced tumbling response and visualization of individual flagella. J Mol Biol. 1974 Apr 15;84(3):399–406. doi: 10.1016/0022-2836(74)90448-3. [DOI] [PubMed] [Google Scholar]
- Manson M. D., Tedesco P., Berg H. C., Harold F. M., Van der Drift C. A protonmotive force drives bacterial flagella. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3060–3064. doi: 10.1073/pnas.74.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCuskey R. S. In vivo microscopy of internal organs. Prog Clin Biol Res. 1981;59B:79–87. [PubMed] [Google Scholar]
- Paster B. J., Canale-Parola E. Involvement of periplasmic fibrils in motility of spirochetes. J Bacteriol. 1980 Jan;141(1):359–364. doi: 10.1128/jb.141.1.359-364.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGER J. M., PLOTZ C. M., GOLDBERG R. THE DETECTION OF ANTI-GLOBULIN FACTORS UTILIZING PRE-COATED LATEX ARTICLES. Arthritis Rheum. 1965 Apr;8:194–202. doi: 10.1002/art.1780080203. [DOI] [PubMed] [Google Scholar]
- Shioi J. I., Matsuura S., Imae Y. Quantitative measurements of proton motive force and motility in Bacillus subtilis. J Bacteriol. 1980 Dec;144(3):891–897. doi: 10.1128/jb.144.3.891-897.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Flagellar rotation and the mechanism of bacterial motility. Nature. 1974 May 3;249(452):73–74. doi: 10.1038/249073a0. [DOI] [PubMed] [Google Scholar]
- Spudich J. L., Koshland D. E., Jr Specific inactivator of flagellar reversal in Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):442–447. doi: 10.1128/jb.139.2.442-447.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui S., Amidror I. Digital low-pass differentiation for biological signal processing. IEEE Trans Biomed Eng. 1982 Oct;29(10):686–693. doi: 10.1109/TBME.1982.324861. [DOI] [PubMed] [Google Scholar]




