Abstract
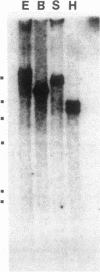
A precisely ordered crystalline array is found on the surface of the bacterium Caulobacter crescentus CB15. Using an immunological assay, we identified recombinant bacteriophage clones expressing the predominant protein of this structure from a lambda 1059 library of C. crescentus CB15 DNA. A single 4.4-kilobase HindIII fragment encoded a polypeptide whose antigenic determinants, molecular weight, and peculiar solubilization properties were identical with those of the authentic predominant polypeptide (130K) of the surface array. The 130K protein was produced as a discrete product as a result of gene transcription initiated from a lambda promoter; several experiments suggested that the Caulobacter promoter for this gene is not efficiently recognized by the Escherichia coli transcription machinery. Genomic Southern analysis revealed a single copy of the 130K protein gene per genome. The 130K protein gene was hybridized with DNA of two closely related laboratory strains of C. crescentus which have lost their ability to produce a surface array. One of these strains, CB2, possesses an homologous copy of the 130K gene, whereas DNA from the other strain, CB13B1a, showed a lesser degree of hybridization to the 130K gene probe; genomic fragments which did hybridize were of different sizes in CB13 as compared with those of CB15. These findings are discussed in relation to studies of the surface array function and its role in cellular morphogenesis in this stalk-forming bacterium.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Henning U., Schwarz H., Chen R. Radioimmunological screening method for specific membrane proteins. Anal Biochem. 1979 Aug;97(1):153–157. doi: 10.1016/0003-2697(79)90339-7. [DOI] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L., Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. doi: 10.1073/pnas.77.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murialdo H., Siminovitch L. The morphogenesis of bacteriophage lambda. IV. Identification of gene products and control of the expression of the morphogenetic information. Virology. 1972 Jun;48(3):785–823. doi: 10.1016/0042-6822(72)90162-6. [DOI] [PubMed] [Google Scholar]
- Ohta N., Chen L. S., Newton A. Isolation and expression of cloned hook protein gene from Caulobacter crescentus. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4863–4867. doi: 10.1073/pnas.79.16.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindexter J. S., Hornack P. R., Armstrong P. A. Intracellular development of a large DNA bacteriophage lytic for Caulobacter crescentus. Arch Mikrobiol. 1967;59(1):237–246. doi: 10.1007/BF00406337. [DOI] [PubMed] [Google Scholar]
- Poindexter J. S. Selection for nonbuoyant morphological mutants of Caulobacter crescentus. J Bacteriol. 1978 Sep;135(3):1141–1145. doi: 10.1128/jb.135.3.1141-1145.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. ISOLATION OF THE lambda PHAGE REPRESSOR. Proc Natl Acad Sci U S A. 1967 Feb;57(2):306–313. doi: 10.1073/pnas.57.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Agabian N. Cell surface patterning and morphogenesis: biogenesis of a periodic surface array during Caulobacter development. J Cell Biol. 1982 Oct;95(1):41–49. doi: 10.1083/jcb.95.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Grano D. A., Glaeser R. M., Agabian N. Periodic surface array in Caulobacter crescentus: fine structure and chemical analysis. J Bacteriol. 1981 Jun;146(3):1135–1150. doi: 10.1128/jb.146.3.1135-1150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Hermodson M., Agabian N. Caulobacter crescentus pilin. Purification, chemical characterization, and NH2-terminal amino acid sequence of a structural protein regulated during development. J Biol Chem. 1981 Mar 25;256(6):3092–3097. [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stoker N. G., Pratt J. M., Spratt B. G. Identification of the rodA gene product of Escherichia coli. J Bacteriol. 1983 Aug;155(2):854–859. doi: 10.1128/jb.155.2.854-859.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Müller-Hill B., Abrutsch U., Aichele G., Overath P. Amplification of the lactose carrier protein in Escherichia coli using a plasmid vector. Mol Gen Genet. 1978 Feb 27;159(3):239–248. doi: 10.1007/BF00268260. [DOI] [PubMed] [Google Scholar]
- West D., Lagenaur C., Agabian N. Isolation and characterization of Caulobacter crecentus bacteriophage phi Cd1. J Virol. 1976 Feb;17(2):568–575. doi: 10.1128/jvi.17.2.568-575.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler M. E., Schoenlein P. V., Ross C. M., Barrett J. T., Ely B. Genetic and physical analyses of Caulobacter crescentus trp genes. J Bacteriol. 1984 Oct;160(1):279–287. doi: 10.1128/jb.160.1.279-287.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]