Abstract
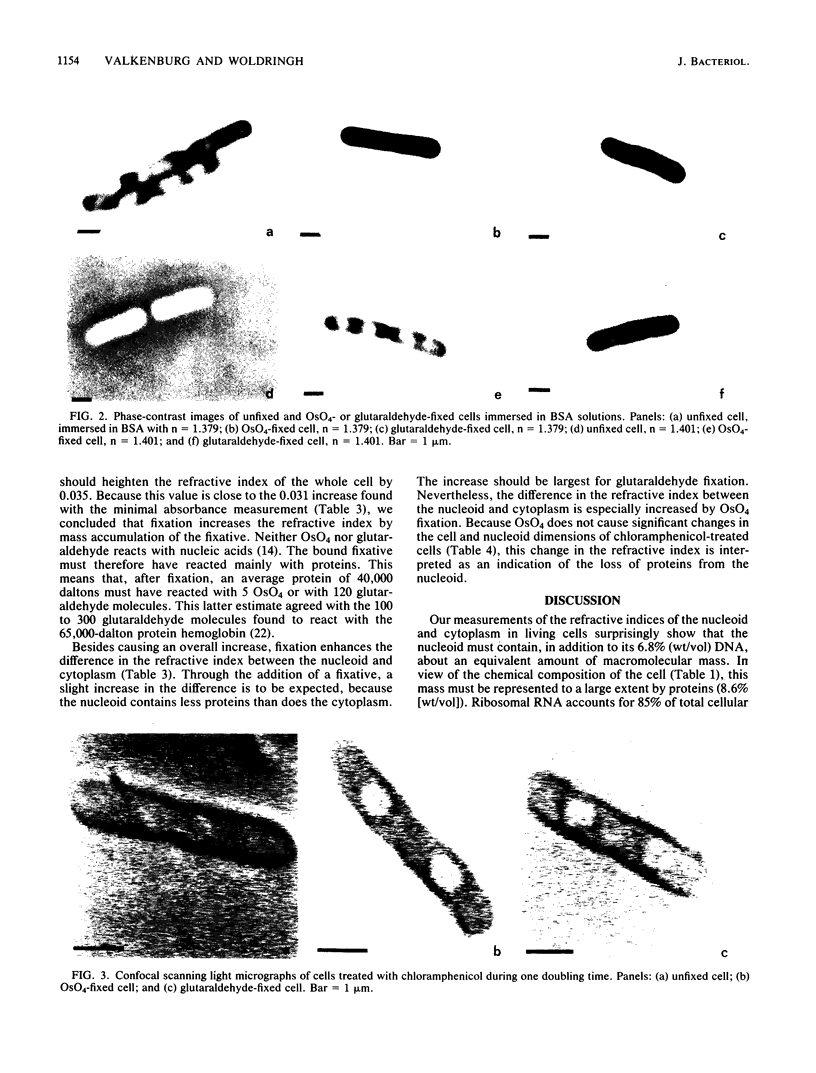
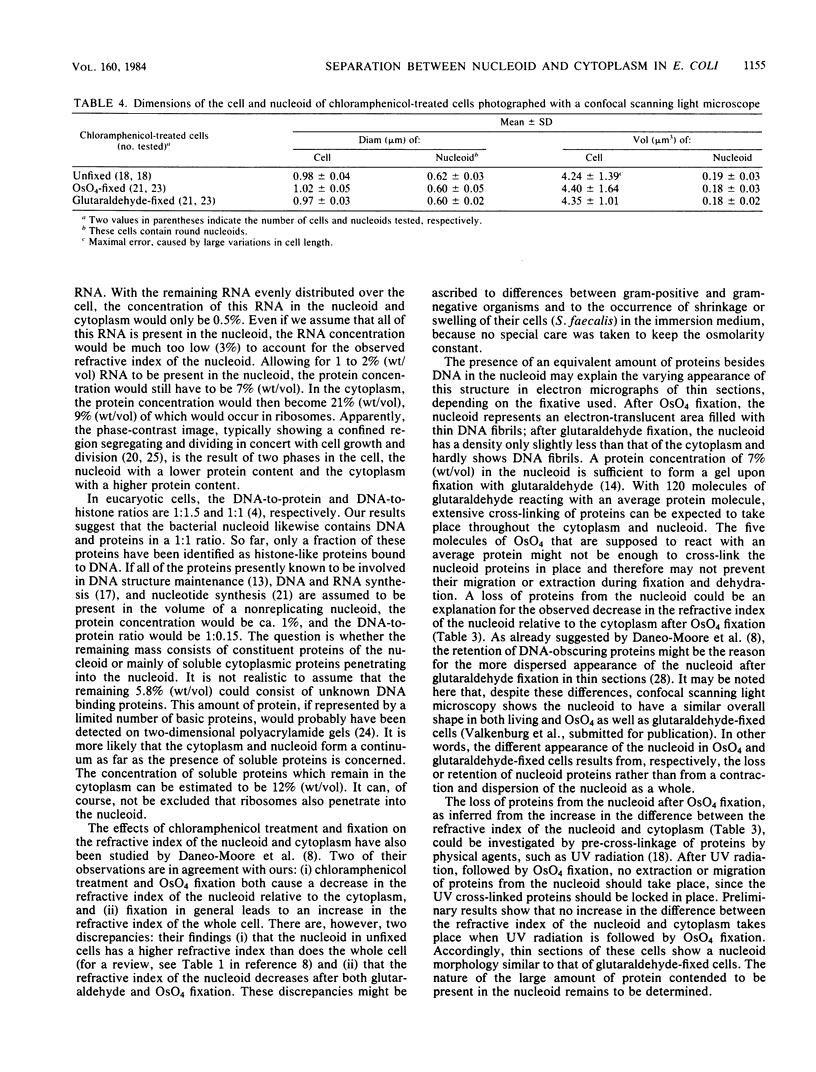
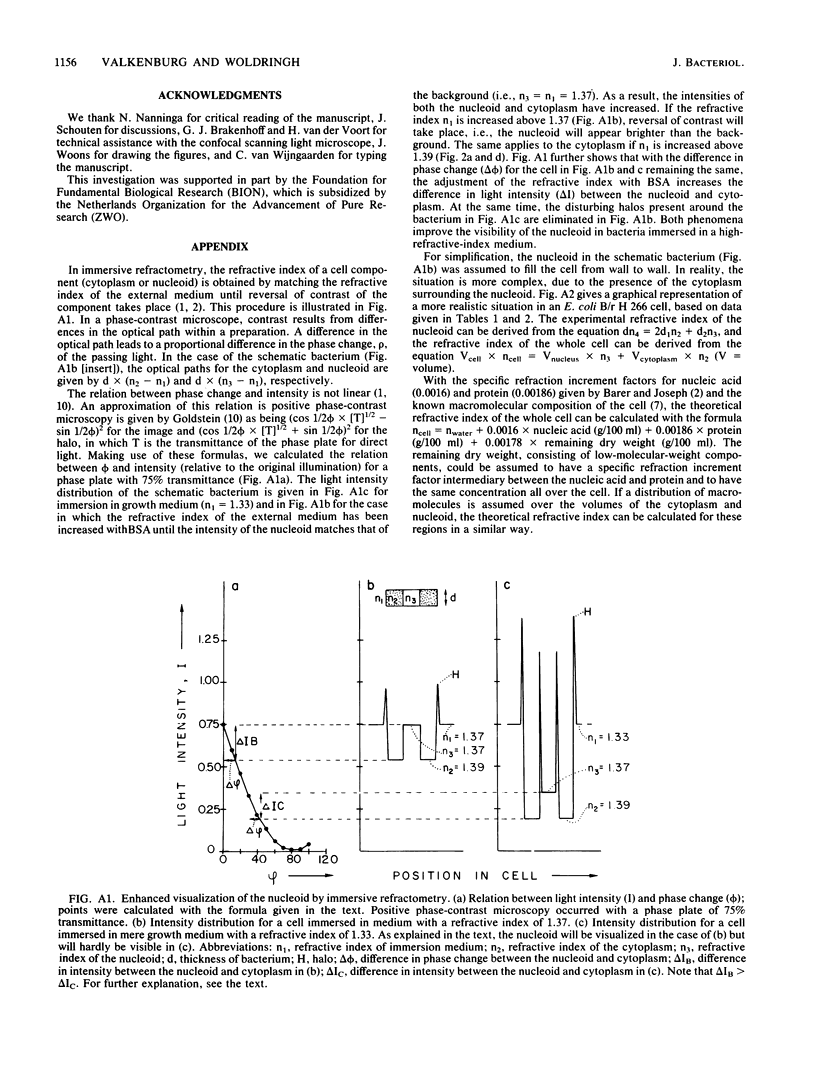
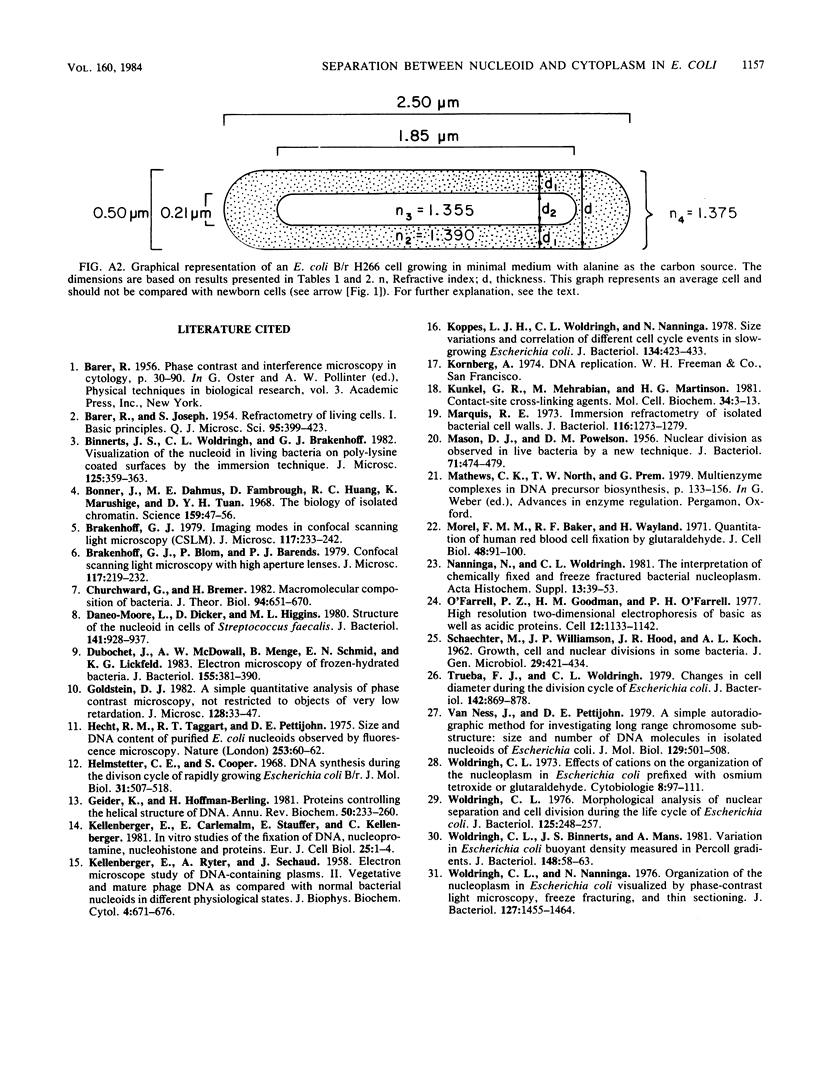
The refractive indices of nucleoid and cytoplasm in Escherichia coli were derived theoretically and experimentally. For the theoretical estimates, we made use of the known macromolecular composition of E. coli B/r (G. Churchward and H. Bremer, J. Theor. Biol. 94:651-670, 1982) and of estimates of cell and nucleoid volumes. These were obtained from micrographs of living bacteria made with a confocal scanning light microscope. The theoretical values were calculated, assuming that all DNA occurred in the nucleoid and that all protein and RNA occurred in the cytoplasm. Comparison with experimental refractive index values directly obtained by immersive refractometry showed that, besides its DNA, the nucleoid must contain an additional amount of solids equivalent to 8.6% (wt/vol) protein. With the nucleoid containing 6.8% (wt/vol) DNA and 8.6% (wt/vol) protein and the cytoplasm containing 21% (wt/vol) protein and 4% (wt/vol) RNA, a mass difference is obtained, which accounts for the phase separation observed between the nucleoid and cytoplasm in living cells by phase-contrast microscopy. The decrease in the refractive index of the nucleoid relative to that of the cytoplasm observed upon, for instance, OsO4 fixation was interpreted as being indicative of the loss of protein content in the nucleoid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binnerts J. S., Woldringh C. L., Brakenhoff G. J. Visualization of the nucleoid in living bacteria on poly-lysine coated surfaces by the immersion technique. J Microsc. 1982 Mar;125(Pt 3):359–363. doi: 10.1111/j.1365-2818.1982.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Bonner J., Dahmus M. E., Fambrough D., Huang R. C., Marushige K., Tuan D. Y. The Biology of Isolated Chromatin: Chromosomes, biologically active in the test tube, provide a powerful tool for the study of gene action. Science. 1968 Jan 5;159(3810):47–56. doi: 10.1126/science.159.3810.47. [DOI] [PubMed] [Google Scholar]
- Churchward G., Bremer H., Young R. Macromolecular composition of bacteria. J Theor Biol. 1982 Feb 7;94(3):651–670. doi: 10.1016/0022-5193(82)90305-8. [DOI] [PubMed] [Google Scholar]
- Daneo-Moore L., Dicker D., Higgins M. L. Structure of the nucleoid in cells of Streptococcus faecalis. J Bacteriol. 1980 Feb;141(2):928–937. doi: 10.1128/jb.141.2.928-937.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubochet J., McDowall A. W., Menge B., Schmid E. N., Lickfeld K. G. Electron microscopy of frozen-hydrated bacteria. J Bacteriol. 1983 Jul;155(1):381–390. doi: 10.1128/jb.155.1.381-390.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geider K., Hoffmann-Berling H. Proteins controlling the helical structure of DNA. Annu Rev Biochem. 1981;50:233–260. doi: 10.1146/annurev.bi.50.070181.001313. [DOI] [PubMed] [Google Scholar]
- Goldstein D. J. A simple quantitative analysis of phase contrast microscopy, not restricted to objects of very low retardation. J Microsc. 1982 Oct;128(Pt 1):33–47. doi: 10.1111/j.1365-2818.1982.tb00435.x. [DOI] [PubMed] [Google Scholar]
- Hecht R. M., Taggart R. T., Pettijohn D. E. Size and DNA content of purfied E. coli nucleoids observed by fluorencence microscopy. Nature. 1975 Jan 3;253(5486):60–62. doi: 10.1038/253060a0. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. DNA synthesis during the division cycle of rapidly growing Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):507–518. doi: 10.1016/0022-2836(68)90424-5. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger E., Carlemalm E., Stauffer E., Kellenberger C., Wunderli H. In vitro studies of the fixation of DNA, nucleoprotamine, nucleohistone and proteins. Eur J Cell Biol. 1981 Aug;25(1):1–4. [PubMed] [Google Scholar]
- Koppes L. H., Woldringh C. L., Nanninga N. Size variations and correlation of different cell cycle events in slow-growing Escherichia coli. J Bacteriol. 1978 May;134(2):423–433. doi: 10.1128/jb.134.2.423-433.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel G. R., Mehrabian M., Martinson H. G. Contact-site cross-linking agents. Mol Cell Biochem. 1981 Jan 20;34(1):3–13. doi: 10.1007/BF02354846. [DOI] [PubMed] [Google Scholar]
- MASON D. J., POWELSON D. M. Nuclear division as observed in live bacteria by a new technique. J Bacteriol. 1956 Apr;71(4):474–479. doi: 10.1128/jb.71.4.474-479.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E. Immersion refractometry of isolated bacterial cell walls. J Bacteriol. 1973 Dec;116(3):1273–1279. doi: 10.1128/jb.116.3.1273-1279.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel F. M., Baker R. F., Wayland H. Quantitation of human red blood cell fixation by glutaraldehyde. J Cell Biol. 1971 Jan;48(1):91–100. doi: 10.1083/jcb.48.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanninga N., Woldringh C. L. The interpretation of chemically fixed and freeze-fractured bacterial nucleoplasm. Acta Histochem Suppl. 1981;23:39–53. [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., WILLIAMSON J. P., HOOD J. R., Jr, KOCH A. L. Growth, cell and nuclear divisions in some bacteria. J Gen Microbiol. 1962 Nov;29:421–434. doi: 10.1099/00221287-29-3-421. [DOI] [PubMed] [Google Scholar]
- Trueba F. J., Woldringh C. L. Changes in cell diameter during the division cycle of Escherichia coli. J Bacteriol. 1980 Jun;142(3):869–878. doi: 10.1128/jb.142.3.869-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ness J., Pettijohn D. E. A simple autoradiographic method for investigating long range chromosome substructure: size and number of DNA molecules in isolated nucleoids of Escherichia coli. J Mol Biol. 1979 Apr 15;129(3):501–508. doi: 10.1016/0022-2836(79)90509-6. [DOI] [PubMed] [Google Scholar]
- Woldringh C. L., Binnerts J. S., Mans A. Variation in Escherichia coli buoyant density measured in Percoll gradients. J Bacteriol. 1981 Oct;148(1):58–63. doi: 10.1128/jb.148.1.58-63.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldringh C. L. Morphological analysis of nuclear separation and cell division during the life cycle of Escherichia coli. J Bacteriol. 1976 Jan;125(1):248–257. doi: 10.1128/jb.125.1.248-257.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldringh C. L., Nanninga N. Organization of the nucleoplasm in Escherichia coli visualized by phase-contrast light microscopy, freeze fracturing, and thin sectioning. J Bacteriol. 1976 Sep;127(3):1455–1464. doi: 10.1128/jb.127.3.1455-1464.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]