Abstract
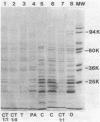
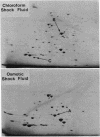
We introduce a method by which periplasmic proteins can be released rapidly, simply, and quantitatively by treating cells with chloroform. All the amino acid-binding proteins tested maintained their activity during chloroform treatment. This method makes practical the analysis of the periplasmic protein complement of a large number of strains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Hogg R. W. "In vivo" detection of L-arabinose-binding protein, CRM-negative mutants. J Bacteriol. 1971 Feb;105(2):604–608. doi: 10.1128/jb.105.2.604-608.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S. G., Ames G. F. The histidine-binding protein J, a histidine transport component, has two different functional sites. J Biol Chem. 1974 Nov 10;249(21):6976–6983. [PubMed] [Google Scholar]
- Kustu S. G., McFarland N. C., Hui S. P., Esmon B., Ames G. F. Nitrogen control of Salmonella typhimurium: co-regulation of synthesis of glutamine synthetase and amino acid transport systems. J Bacteriol. 1979 Apr;138(1):218–234. doi: 10.1128/jb.138.1.218-234.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S. G., McKereghan K. Mutations affecting glutamine synthetase activity in Salmonella typhimurium. J Bacteriol. 1975 Jun;122(3):1006–1016. doi: 10.1128/jb.122.3.1006-1016.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S., Burton D., Garcia E., McCarter L., McFarland N. Nitrogen control in Salmonella: regulation by the glnR and glnF gene products. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4576–4580. doi: 10.1073/pnas.76.9.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lever J. E. Quantitative assay of the binding of small molecules to protein: comparison of dialysis and membrane filter assays. Anal Biochem. 1972 Nov;50(1):73–83. doi: 10.1016/0003-2697(72)90487-3. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R., Ginsburg A., Ciardi J. E., Yeh J., Hennig S. B., Shapiro B. M. Multiple molecular forms of glutamine synthetase produced by enzyme catalyzed adenylation and deadenylylation reactions. Adv Enzyme Regul. 1970;8:99–118. doi: 10.1016/0065-2571(70)90011-7. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Wei G. R., Kustu S. Glutamine auxotrophs with mutations in a nitrogen regulatory gene, ntrC, that is near glnA. Mol Gen Genet. 1981;183(2):392–399. doi: 10.1007/BF00270646. [DOI] [PubMed] [Google Scholar]