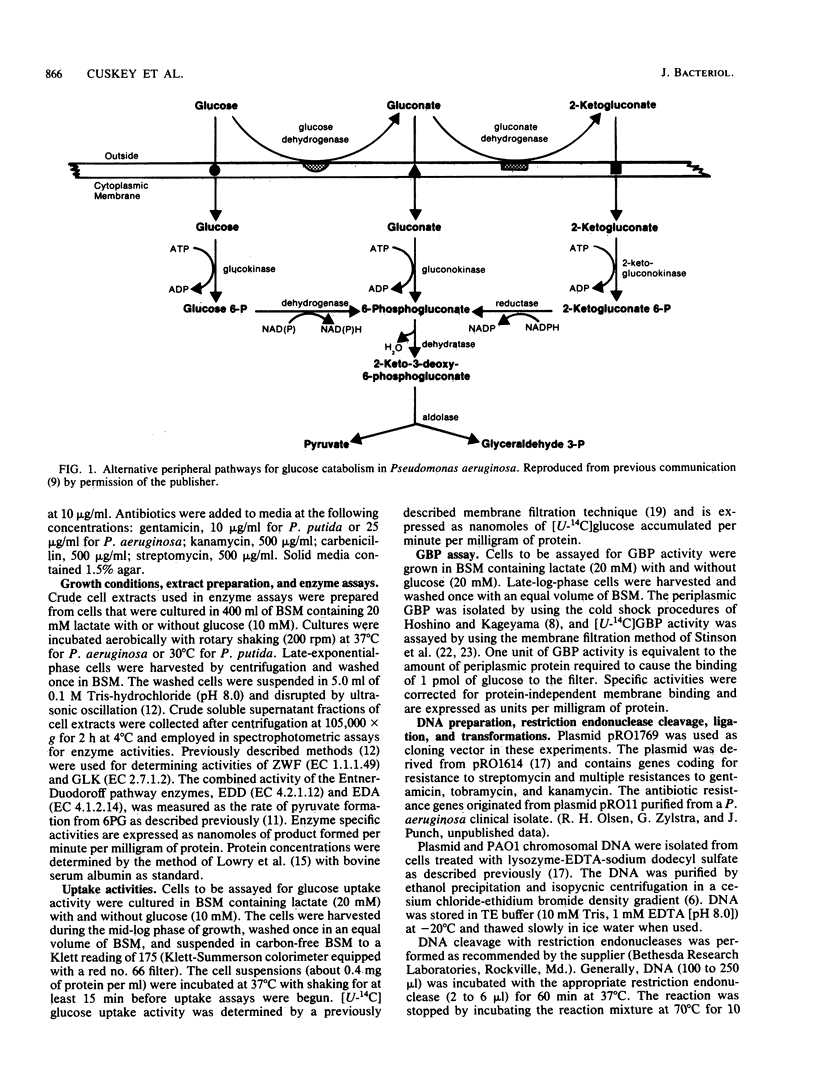
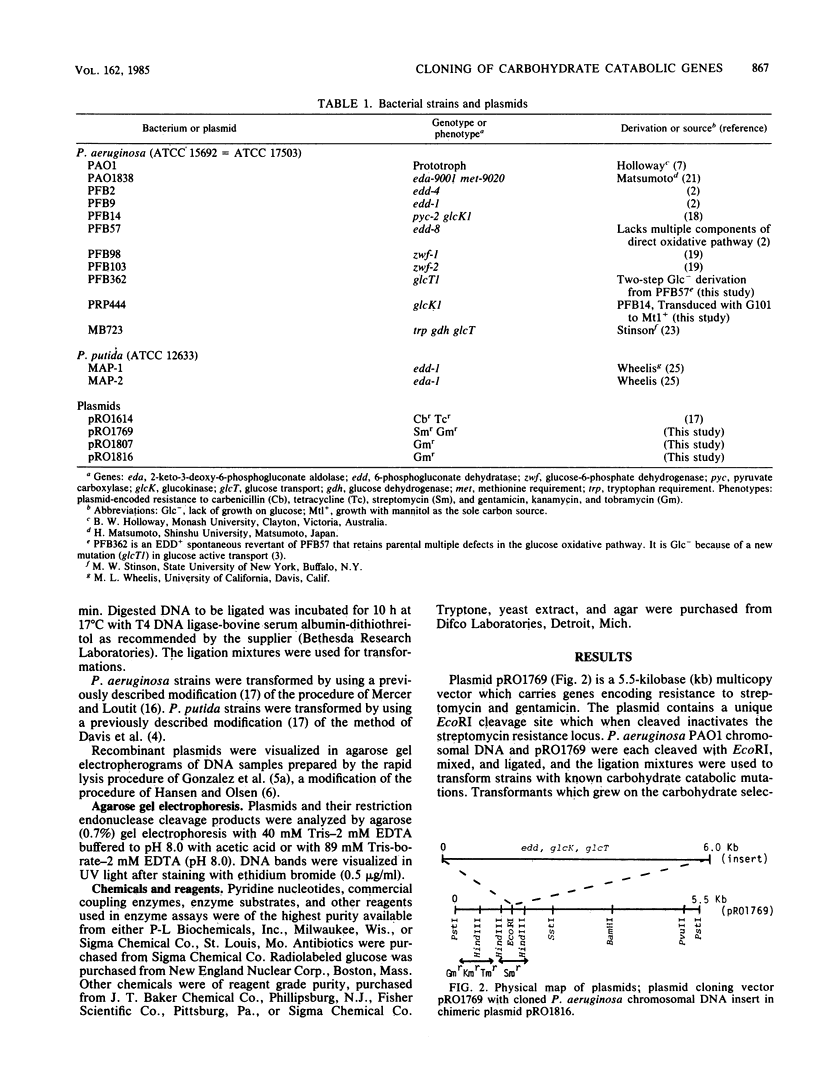
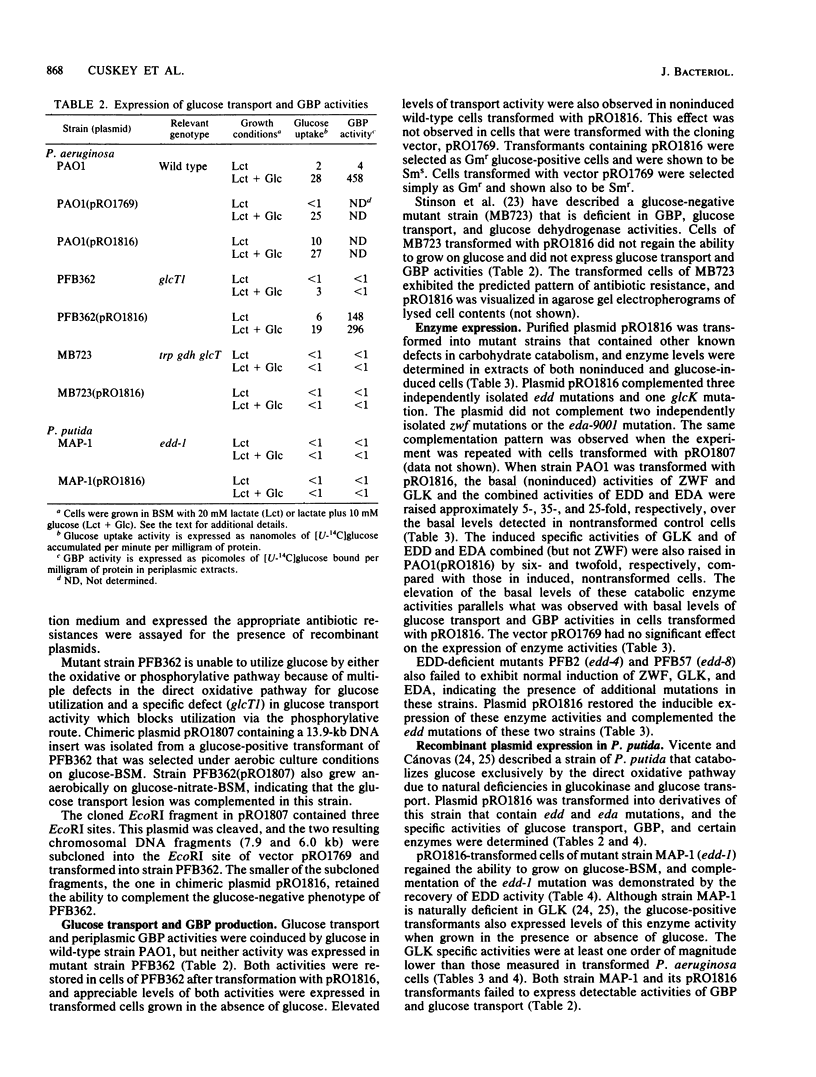
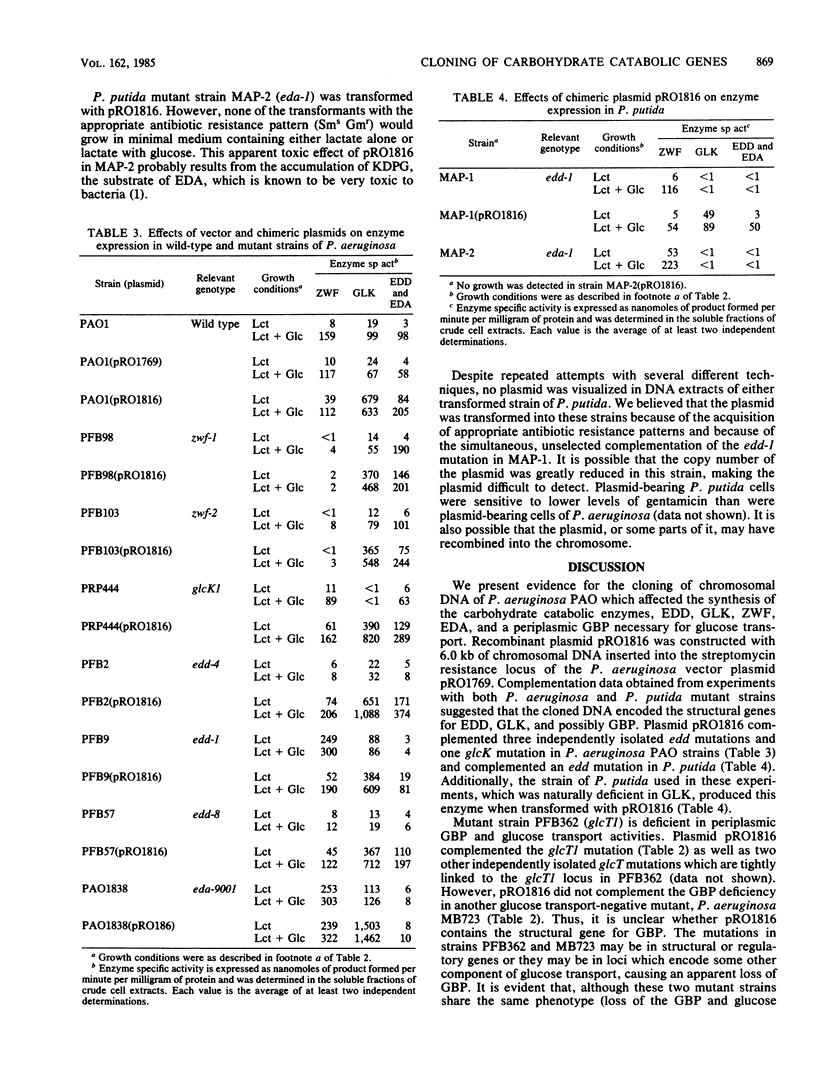
Abstract
A 6.0-kilobase EcoRI fragment of the Pseudomonas aeruginosa PAO chromosome containing a cluster of genes specifying carbohydrate catabolism was cloned into the multicopy plasmid pRO1769. The vector contains a unique EcoRI site for cloning within a streptomycin resistance determinant and a selectable gene encoding gentamicin resistance. Mutants of P. aeruginosa PAO transformed with the chimeric plasmid pRO1816 regained the ability to grow on glucose, and the following deficiencies in enzyme or transport activities corresponding to the specific mutations were complemented: glcT1, glucose transport and periplasmic glucose-binding protein; glcK1, glucokinase; and edd-1, 6-phosphogluconate dehydratase. Two other carbohydrate catabolic markers that are cotransducible with glcT1 and edd-1 were not complemented by plasmid pRO1816: zwf-1, glucose-6-phosphate dehydrogenase; and eda-9001, 2-keto-3-deoxy-6-phosphogluconate aldolase. However, all five of these normally inducible activities were expressed at markedly elevated basal levels when transformed cells of prototrophic strain PAO1 were grown without carbohydrate inducer. Vector plasmid pRO1769 had no effect on the expression of these activities in transformed mutant or wild-type cells. Thus, the chromosomal insert in pRO1816 contains the edd and glcK structural genes, at least one gene (glcT) that is essential for expression of the glucose active transport system, and other loci that regulate the expression of the five clustered carbohydrate catabolic genes. The insert in pRO1816 also complemented the edd-1 mutation in a glucose-negative Pseudomonas putida mutant but not the eda-1 defect in another mutant. Moreover, pRO1816 caused the expression of high specific activities of glucokinase, an enzyme that is naturally lacking in these strains of Pseudomonas putida.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allenza P., Lessie T. G. Pseudomonas cepacia mutants blocked in the Entner-Doudoroff pathway. J Bacteriol. 1982 Jun;150(3):1340–1347. doi: 10.1128/jb.150.3.1340-1347.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins W. T., Feary T. W., Phibbs P. V., Jr 6-Phosphogluconate dehydratase deficiency in pleiotropic carbohydrate-negative mutant strains of Pseudomonas aeruginosa. J Bacteriol. 1975 Mar;121(3):942–949. doi: 10.1128/jb.121.3.942-949.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuskey S. M., Phibbs P. V., Jr Chromosomal mapping of mutations affecting glycerol and glucose catabolism in Pseudomonas aeruginosa PAO. J Bacteriol. 1985 Jun;162(3):872–880. doi: 10.1128/jb.162.3.872-880.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham D. R., Phibbs P. V., Jr Fractionation and characterization of the phosphoenolpyruvate: fructose 1-phosphotransferase system from Pseudomonas aeruginosa. J Bacteriol. 1982 Feb;149(2):534–541. doi: 10.1128/jb.149.2.534-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Morgan A. F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979 Mar;43(1):73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T., Kageyama M. Purification and properties of a binding protein for branched-chain amino acids in Pseudomonas aeruginosa. J Bacteriol. 1980 Mar;141(3):1055–1063. doi: 10.1128/jb.141.3.1055-1063.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. C., Phibbs P. V., Jr Failure of Pseudomonas aeruginosa to form membrane-associated glucose dehydrogenase activity during anaerobic growth with nitrate. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1393–1399. doi: 10.1016/s0006-291x(81)80166-0. [DOI] [PubMed] [Google Scholar]
- Hylemon P. B., Krieg N. R., Phibbs P. V., Jr Transport and catabolism of D-fructose by Spirillum itersomii. J Bacteriol. 1974 Jan;117(1):144–150. doi: 10.1128/jb.117.1.144-150.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon P. B., Phibbs P. V., Jr Independent regulation of hexose catabolizing enzymes and glucose transport activity in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1041–1048. doi: 10.1016/0006-291x(72)90813-3. [DOI] [PubMed] [Google Scholar]
- KOVACHEVICH R., WOOD W. A. Carbohydrate metabolism by Pseudomonas fluorescens. III. Purification and properties of a 6-phosphogluconate dehydrase. J Biol Chem. 1955 Apr;213(2):745–756. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lessie T. G., Phibbs P. V., Jr Alternative pathways of carbohydrate utilization in pseudomonads. Annu Rev Microbiol. 1984;38:359–388. doi: 10.1146/annurev.mi.38.100184.002043. [DOI] [PubMed] [Google Scholar]
- Mercer A. A., Loutit J. S. Transformation and transfection of Pseudomonas aeruginosa: effects of metal ions. J Bacteriol. 1979 Oct;140(1):37–42. doi: 10.1128/jb.140.1.37-42.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. H., DeBusscher G., McCombie W. R. Development of broad-host-range vectors and gene banks: self-cloning of the Pseudomonas aeruginosa PAO chromosome. J Bacteriol. 1982 Apr;150(1):60–69. doi: 10.1128/jb.150.1.60-69.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phibbs P. V., Jr, Feary T. W., Blevins W. T. Pyruvate carboxylase deficiency in pleiotropic carbohydrate-negative mutant strains of Pseudomonas aeruginosa. J Bacteriol. 1974 Jun;118(3):999–1009. doi: 10.1128/jb.118.3.999-1009.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phibbs P. V., Jr, McCowen S. M., Feary T. W., Blevins W. T. Mannitol and fructose catabolic pathways of Pseudomonas aeruginosa carbohydrate-negative mutants and pleiotropic effects of certain enzyme deficiencies. J Bacteriol. 1978 Feb;133(2):717–728. doi: 10.1128/jb.133.2.717-728.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehl R. A., Feary T. W., Phibbs P. V., Jr Clustering of mutations affecting central pathway enzymes of carbohydrate catabolism in Pseudomonas aeruginosa. J Bacteriol. 1983 Dec;156(3):1123–1129. doi: 10.1128/jb.156.3.1123-1129.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehl R. A., Phibbs P. V., Jr Characterization and genetic mapping of fructose phosphotransferase mutations in Pseudomonas aeruginosa. J Bacteriol. 1982 Mar;149(3):897–905. doi: 10.1128/jb.149.3.897-905.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Cohen M. A., Merrick J. M. Isolation of dicarboxylic acid- and glucose-binding proteins from Pseudomonas aeruginosa. J Bacteriol. 1976 Nov;128(2):573–579. doi: 10.1128/jb.128.2.573-579.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Cohen M. A., Merrick J. M. Purification and properties of the periplasmic glucose-binding protein of Pseudomonas aeruginosa. J Bacteriol. 1977 Aug;131(2):672–681. doi: 10.1128/jb.131.2.672-681.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente M., Cánovas J. L. Glucolysis in Pseudomonas putida: physiological role of alternative routes from the analysis of defective mutants. J Bacteriol. 1973 Nov;116(2):908–914. doi: 10.1128/jb.116.2.908-914.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente M., Cánovas J. L. Regulation of the glucolytic enzymes in Pseudomonas putida. Arch Mikrobiol. 1973 Oct 4;93(1):53–64. doi: 10.1007/BF00666080. [DOI] [PubMed] [Google Scholar]



