Abstract
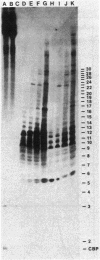
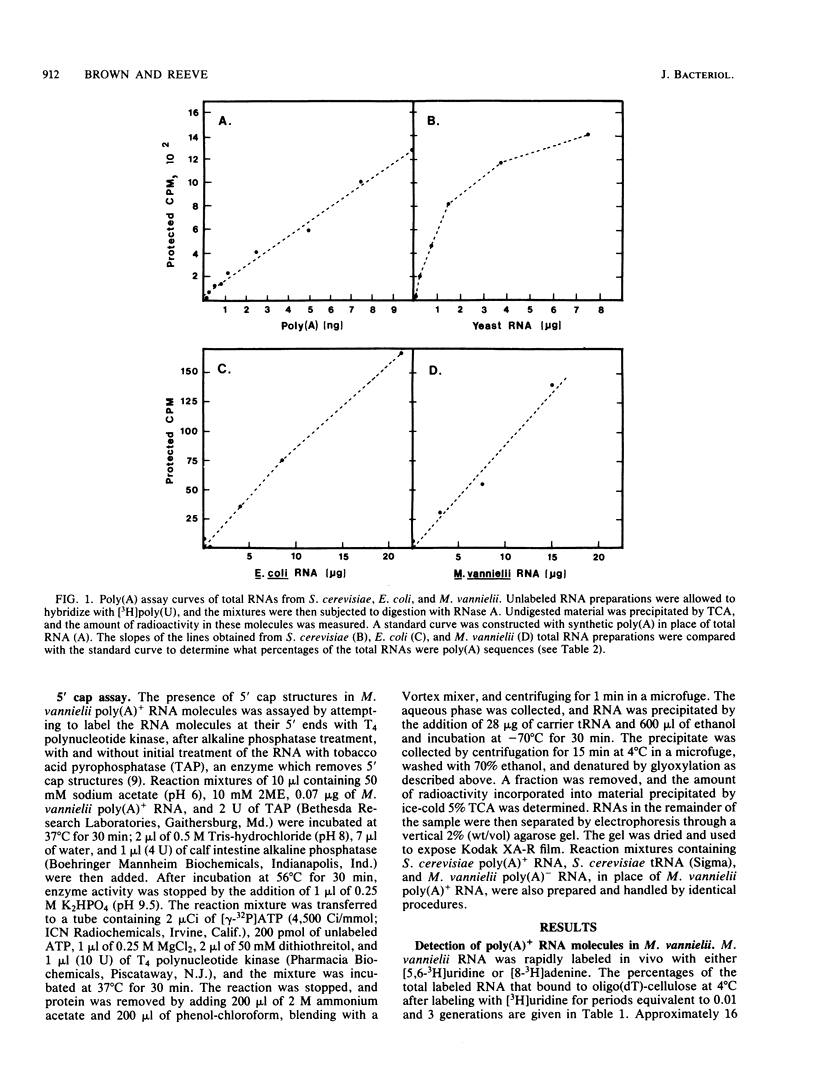
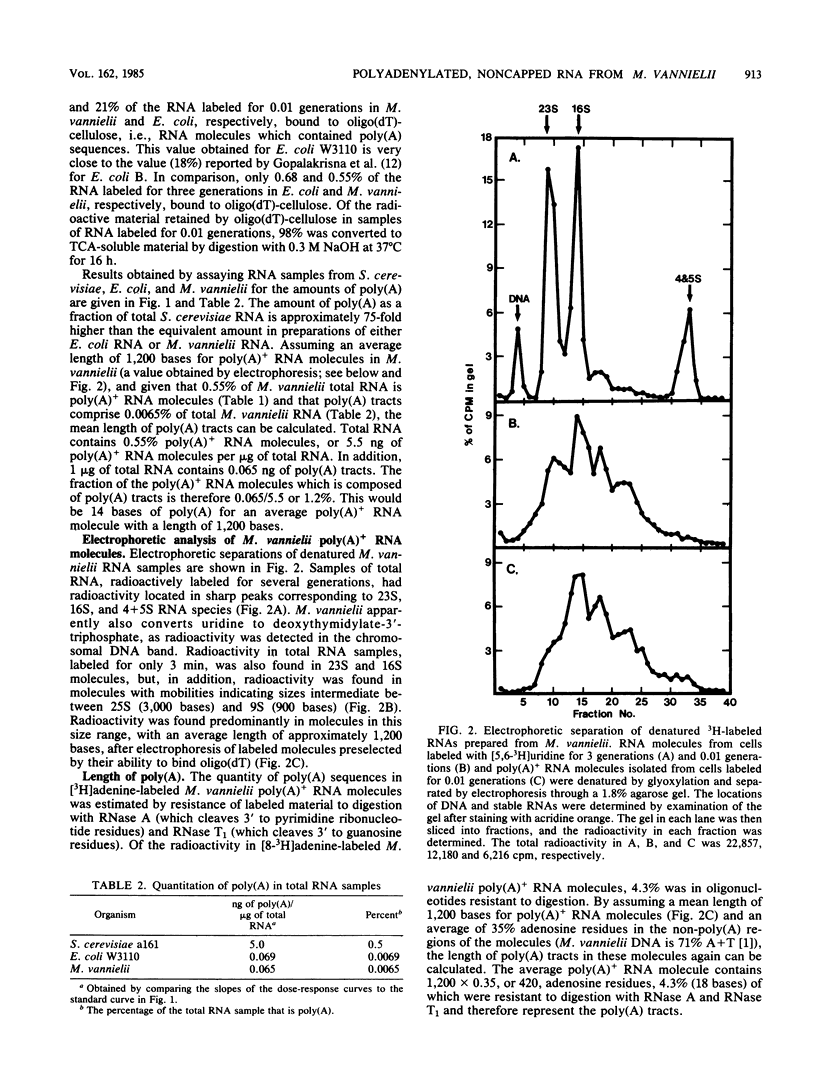
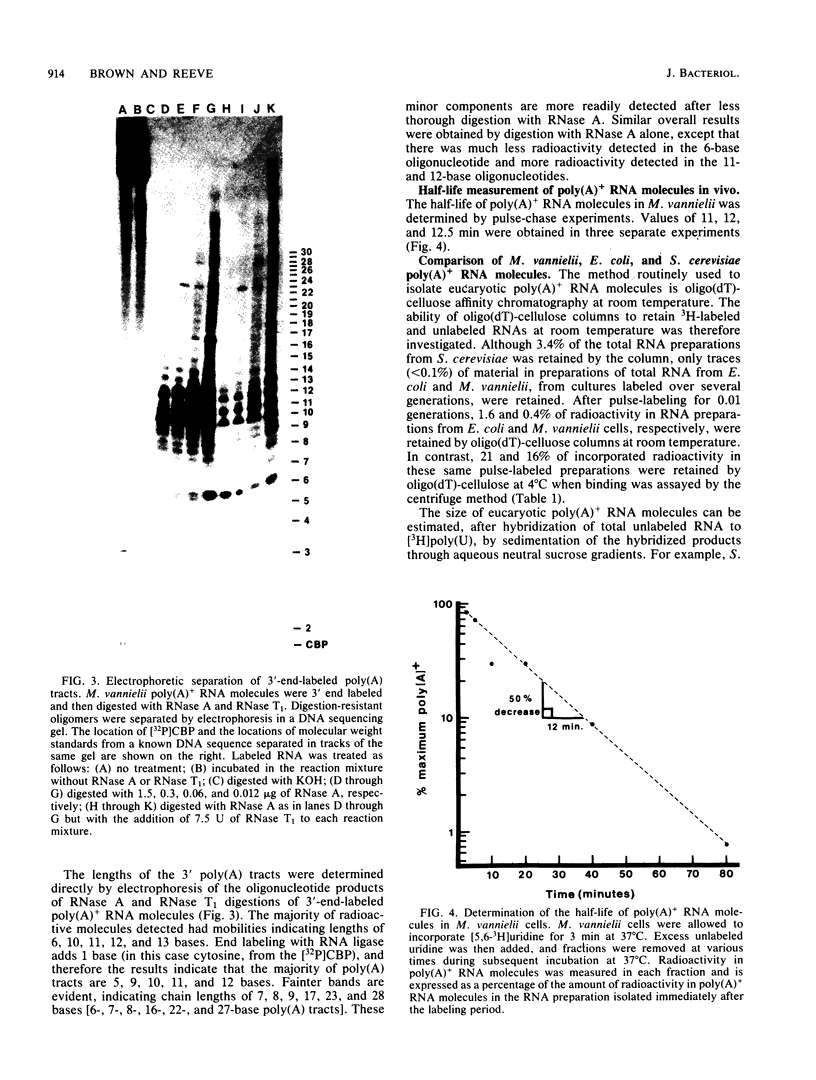
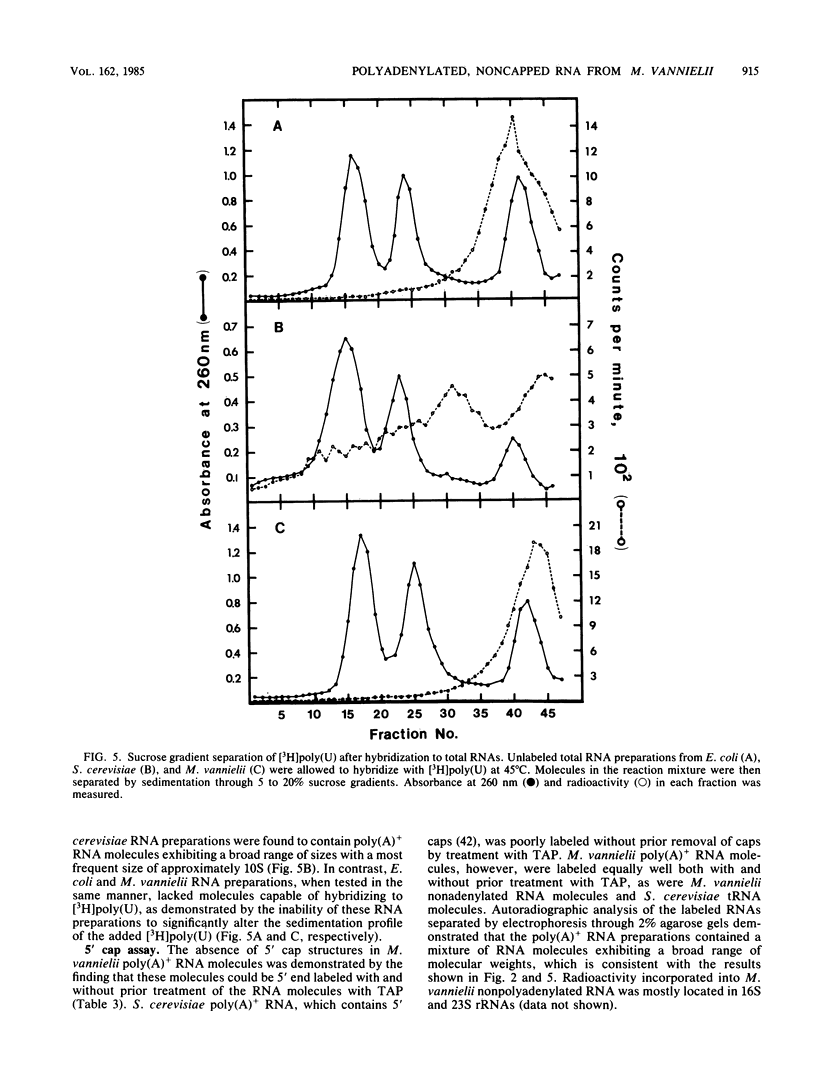
Polyadenylated [poly(A)+] RNA molecules have been isolated from Methanococcus vannielii by oligodeoxythymidylate-cellulose affinity chromatography at 4 degrees C. Approximately 16% of the label in RNA isolated from cultures allowed to incorporate [3H]uridine for 3 min at 37 degrees C was poly(A)+ RNA. In contrast, less than 1% of the radioactivity in RNA labeled over a period of several generations was contained in poly(A)+ RNA molecules. Electrophoretic separation of poly(A)+ RNA molecules showed a heterogeneous population with mobilities indicative of sizes ranging from 900 to 3,000 bases in length. The population of poly(A)+ RNA molecules was found to have a half-life in vivo of approximately 12 min. Polyadenylate [poly(A)] tracts were isolated by digestion with RNase A and RNase T1 after 3' end labeling of the poly(A)+ RNA with RNA ligase. These radioactively labeled poly(A) oligonucleotides were shown by electrophoresis through DNA sequencing gels to average 10 bases in length, with major components of 5, 9, 10, 11, and 12 bases. The lengths of these poly(A) sequences are in agreement with estimates obtained from RNase A and RNase T1 digestions of [3H]adenine-labeled poly(A)+ RNA molecules. Poly(A)+ RNA molecules from M. vannielii were labeled at their 5' termini with T4 polynucleotide kinase after dephosphorylation with calf intestine alkaline phosphatase. Pretreatment of the RNA molecules with tobacco acid pyrophosphatase did not increase the amount of phosphate incorporated into poly(A)+ RNA molecules by polynucleotide kinase, indicating that the poly(A)+ RNA molecules did not have modified bases (caps) at their 5' termini. The relatively short poly(A) tracts, the lack of 5' cap structures, and the instability of the poly(A)+ RNA molecules isolated from M. vannielii indicate that these archaebacterial poly(A)+ RNAs more closely resemble eubacterial mRNAs than eucaryotic mRNAs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. K. 5'-terminal cap structure in eucaryotic messenger ribonucleic acids. Microbiol Rev. 1980 Jun;44(2):175–205. doi: 10.1128/mr.44.2.175-205.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birky C. W., Jr, Demko C. A., Perlman P. S., Strausberg R. Uniparental inheritance of mitochondrial genes in yeast: dependence on input bias of mitochondrial DNA and preliminary investigations of the mechanism. Genetics. 1978 Aug;89(4):615–651. doi: 10.1093/genetics/89.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Furuichi Y., Muthukrishnan S., Shatkin A. J. Ribosome binding to reovirus mRNA in protein synthesis requires 5' terminal 7-methylguanosine. Cell. 1975 Oct;6(2):185–195. doi: 10.1016/0092-8674(75)90009-4. [DOI] [PubMed] [Google Scholar]
- Brawerman G. Characteristics and significance of the polyadenylate sequence in mammalian messenger RNA. Prog Nucleic Acid Res Mol Biol. 1976;17:117–148. doi: 10.1016/s0079-6603(08)60068-9. [DOI] [PubMed] [Google Scholar]
- Crouch D. H., Ownby J. D., Carr N. G. Polyadenylated RNA in two filamentous cyanobacteria. J Bacteriol. 1983 Nov;156(2):979–982. doi: 10.1128/jb.156.2.979-982.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassarma S., Rajbhandary U. L., Khorana H. G. Bacterio-opsin mRNA in wild-type and bacterio-opsin-deficient Halobacterium halobium strains. Proc Natl Acad Sci U S A. 1984 Jan;81(1):125–129. doi: 10.1073/pnas.81.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn R., McCoy J., Simsek M., Majumdar A., Chang S. H., Rajbhandary U. L., Khorana H. G. The bacteriorhodopsin gene. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6744–6748. doi: 10.1073/pnas.78.11.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Vournakis J. N., Donis-Keller H., Chaconas G., Dougall D. K., Kafatos F. C. End labeling of enzymatically decapped mRNA. Nucleic Acids Res. 1977 Dec;4(12):4165–4174. doi: 10.1093/nar/4.12.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna Y., Langley D., Sarkar N. Detection of high levels of polyadenylate-containing RNA in bacteria by the use of a single-step RNA isolation procedure. Nucleic Acids Res. 1981 Jul 24;9(14):3545–3554. doi: 10.1093/nar/9.14.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna Y., Sarkar N. Characterization of polyadenylate-containing ribonucleic acid from Bacillus subtilis. Biochemistry. 1982 May 25;21(11):2724–2729. doi: 10.1021/bi00540a023. [DOI] [PubMed] [Google Scholar]
- Gu X. R., Nicoghosian K., Cedergren R. J., Wong J. T. Sequences of halobacterial tRNAs and the paucity of U in the first position of their anticodons. Nucleic Acids Res. 1983 Aug 25;11(16):5433–5442. doi: 10.1093/nar/11.16.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth M. E., Shumard D. S., Tatti K. M., Grossman L. I. Rapid purification of yeast mitochondrial DNA in high yield. Biochim Biophys Acta. 1980 Dec 11;610(2):221–228. doi: 10.1016/0005-2787(80)90003-9. [DOI] [PubMed] [Google Scholar]
- Huez G., Marbaix G., Hubert E., Leclercq M., Nudel U., Soreq H., Salomon R., Lebleu B., Revel M., Littauer U. Z. Role of the polyadenylate segment in the translation of globin messenger RNA in Xenopus oocytes. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3143–3146. doi: 10.1073/pnas.71.8.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain I., Tsukagoshi N., Udaka S. Characterization of polyadenylated RNA in a protein-producing bacterium, Bacillus brevis 47. J Bacteriol. 1982 Sep;151(3):1162–1170. doi: 10.1128/jb.151.3.1162-1170.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsch M., Böck A. DNA sequence of the 16S rRNA/23S rRNA intercistronic spacer of two rDNA operons of the archaebacterium Methanococcus vannielii. Nucleic Acids Res. 1983 Nov 11;11(21):7537–7544. doi: 10.1093/nar/11.21.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagramanova V. K., Mankin A. S., Baratova L. A., Bogdanov A. A. The 3'-terminal nucleotide sequence of the Halobacterium halobium 16 S rRNA. FEBS Lett. 1982 Jul 19;144(1):177–180. doi: 10.1016/0014-5793(82)80595-4. [DOI] [PubMed] [Google Scholar]
- Kaine B. P., Gupta R., Woese C. R. Putative introns in tRNA genes of prokaryotes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3309–3312. doi: 10.1073/pnas.80.11.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjan P., Szulmajster J. Isolation and characterization of polyadenylated RNA species from sporulating cells of Bacillus subtilis. Biochem Biophys Res Commun. 1980 Mar 13;93(1):201–208. doi: 10.1016/s0006-291x(80)80266-x. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Ihara M., Yabusaki Y., Nishimura S. Initiator tRNAs from archaebacteria show common unique sequence characteristics. Nature. 1982 Aug 12;298(5875):684–685. doi: 10.1038/298684a0. [DOI] [PubMed] [Google Scholar]
- Lamb M. M., Laird C. D. The size of poly(A)-containing RNAs in Drosophila melanogaster embryos. Biochem Genet. 1976 Apr;14(3-4):357–371. doi: 10.1007/BF00484774. [DOI] [PubMed] [Google Scholar]
- Levenson R., Kernen J., Housman D. Synchronization of MEL cell commitment with cordycepin. Cell. 1979 Dec;18(4):1073–1078. doi: 10.1016/0092-8674(79)90220-4. [DOI] [PubMed] [Google Scholar]
- Littauer U. Z., Soreq H. The regulatory function of poly(A) and adjacent 3' sequences in translated RNA. Prog Nucleic Acid Res Mol Biol. 1982;27:53–83. doi: 10.1016/s0079-6603(08)60597-8. [DOI] [PubMed] [Google Scholar]
- Luehrsen K. R., Nicholson D. E., Eubanks D. C., Fox G. E. An archaebacterial 5S rRNA contains a long insertion sequence. Nature. 1981 Oct 29;293(5835):755–756. doi: 10.1038/293755a0. [DOI] [PubMed] [Google Scholar]
- Majumdar P. K., McFadden B. A. Polyadenylated mRNA from the photosynthetic procaryote Rhodospirillum rubrum. J Bacteriol. 1984 Mar;157(3):795–801. doi: 10.1128/jb.157.3.795-801.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar P. K., Vipparti V. A. Polyadenylated messenger RNAs code for photo reaction center and light-harvesting antenna polypeptides of Rhodospirillum rubrum. FEBS Lett. 1980 Jan 1;109(1):31–33. doi: 10.1016/0014-5793(80)81304-4. [DOI] [PubMed] [Google Scholar]
- Mankin A. S., Teterina N. L., Rubtsov P. M., Baratova L. A., Kagramanova V. K. Putative promoter region of rRNA operon from archaebacterium Halobacterium halobium. Nucleic Acids Res. 1984 Aug 24;12(16):6537–6546. doi: 10.1093/nar/12.16.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemer M., Dubroff L. M., Graham M. Properties of sea urchin embryo messenger RNA containing and lacking poly(A). Cell. 1975 Oct;6(2):171–178. doi: 10.1016/0092-8674(75)90007-0. [DOI] [PubMed] [Google Scholar]
- Ohta N., Sanders M., Newton A. Characterization of unstable poly (A)-RNA in Caulobacter crescentus. Biochim Biophys Acta. 1978 Jan 26;517(1):65–75. doi: 10.1016/0005-2787(78)90034-5. [DOI] [PubMed] [Google Scholar]
- Palatnik C. M., Wilkins C., Jacobson A. Translational control during early Dictyostelium development: possible involvement of poly(A) sequences. Cell. 1984 Apr;36(4):1017–1025. doi: 10.1016/0092-8674(84)90051-5. [DOI] [PubMed] [Google Scholar]
- Sarkar N., Langley D., Paulus H. Isolation and characterization of polyadenylate-containing RNA from Bacillus brevis. Biochemistry. 1978 Aug 22;17(17):3468–3474. doi: 10.1021/bi00610a007. [DOI] [PubMed] [Google Scholar]
- Schultz G. A., Chaconas G., Moore R. L. Polyadenylic acid sequences in the RNA of Hyphomicrobium. J Bacteriol. 1978 Feb;133(2):569–575. doi: 10.1128/jb.133.2.569-575.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Sripati C. E., Groner Y., Warner J. R. Methylated, blocked 5' termini of yeast mRNA. J Biol Chem. 1976 May 25;251(10):2898–2904. [PubMed] [Google Scholar]
- Wich G., Jarsch M., Böck A. Apparent operon for a 5S ribosomal RNA gene and for tRNA genes in the archaebacterium Methanococcus vannielii. Mol Gen Genet. 1984;196(1):146–151. doi: 10.1007/BF00334107. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Klett H. Measurement of polyadenylic acid by hybridization with polyuridylic acid: a source of error due to the lability of tritiated polyuridylic acid in trichloroacetic acid. Anal Biochem. 1978 Nov;91(1):173–179. doi: 10.1016/0003-2697(78)90828-x. [DOI] [PubMed] [Google Scholar]