Abstract
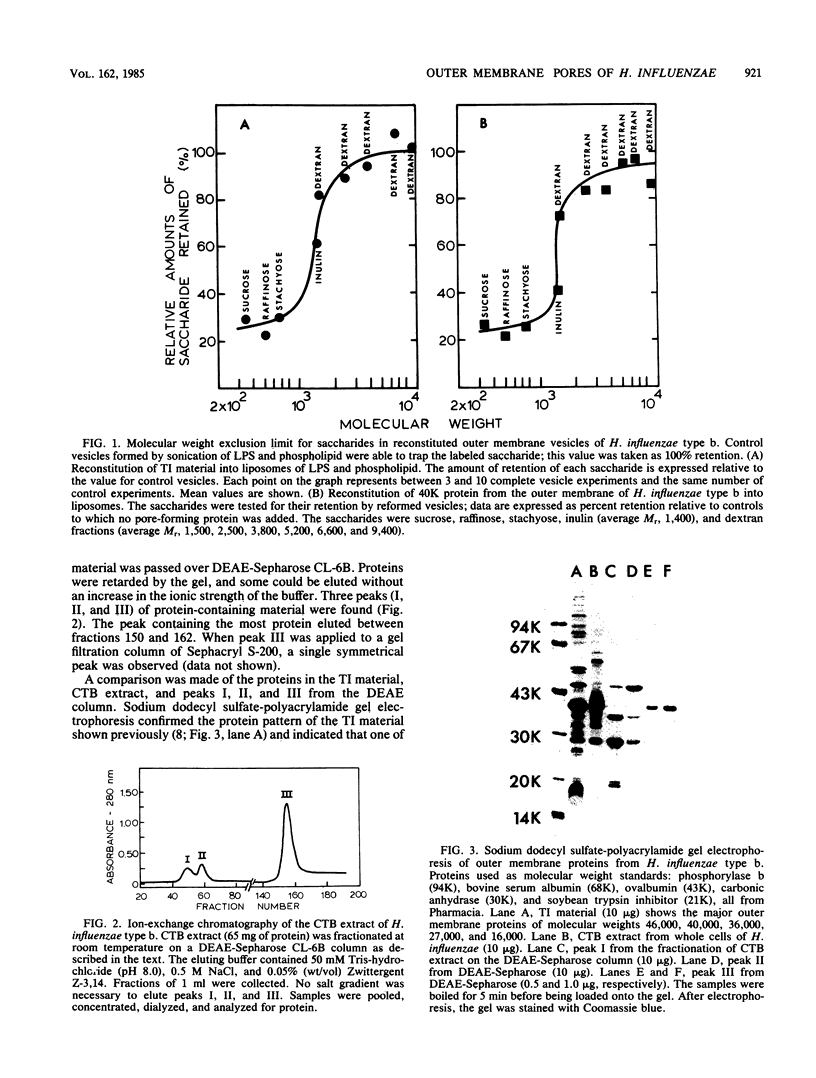
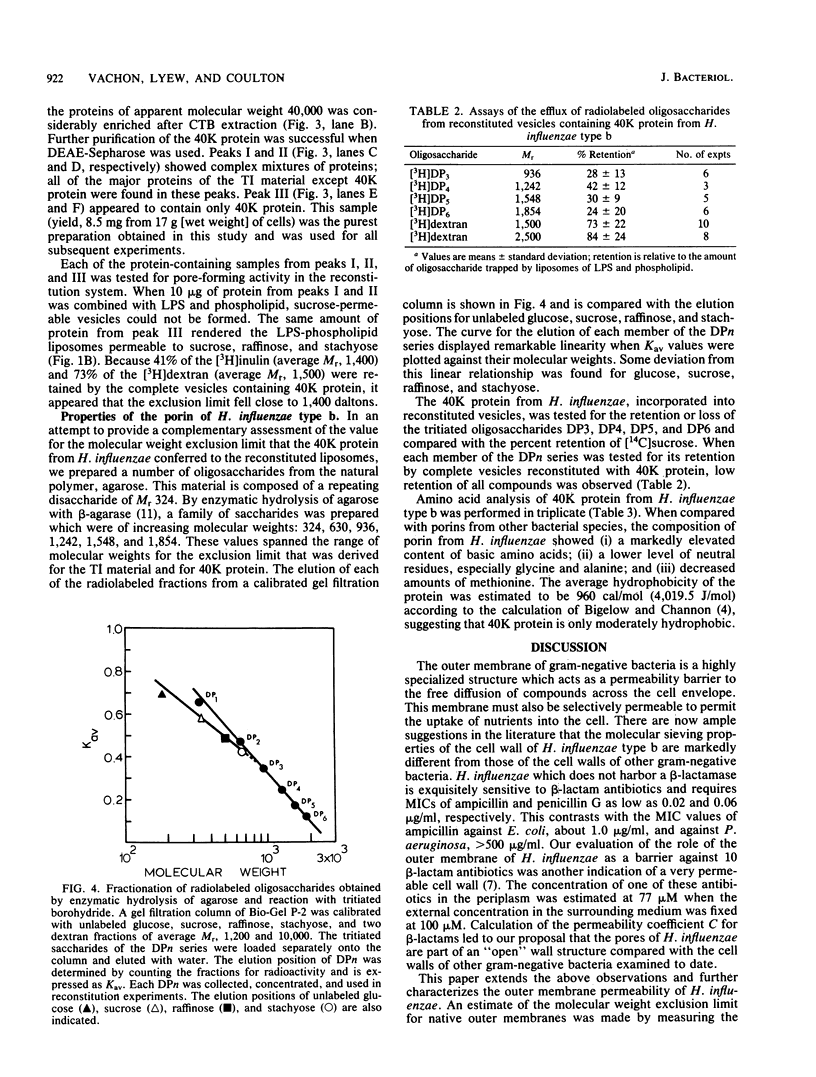
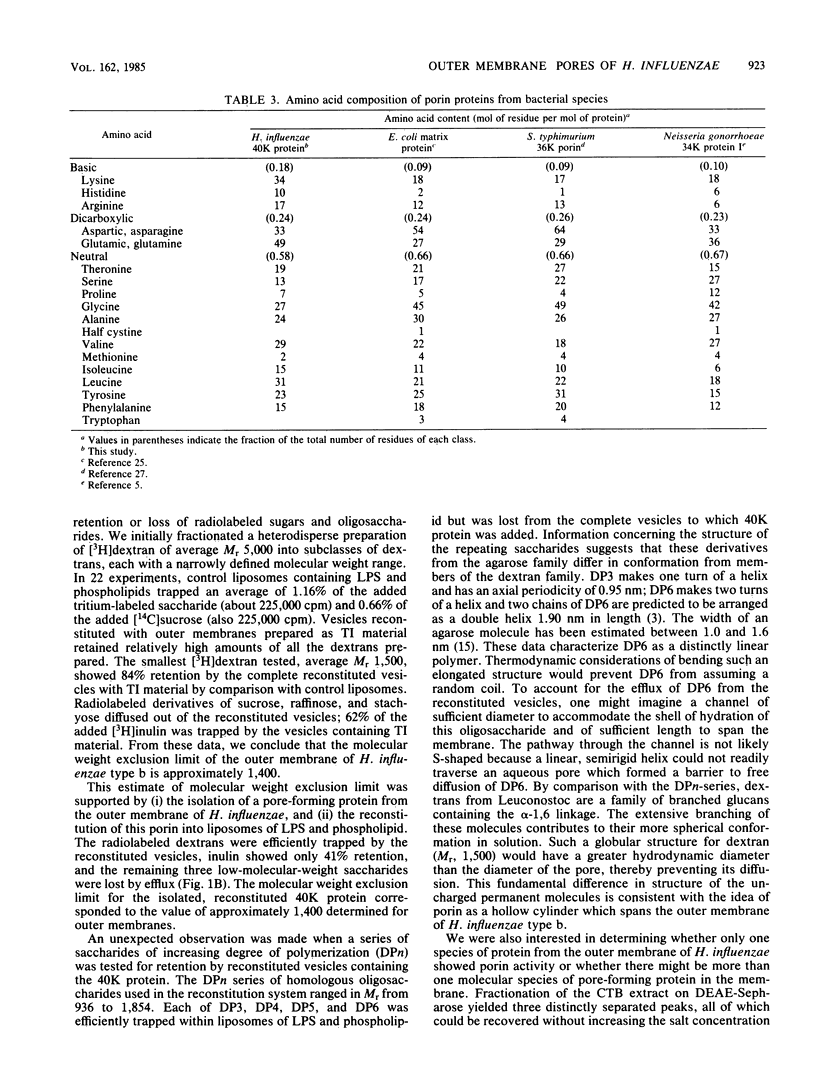
Outer membranes of Haemophilus influenzae type b were fractionated to yield Triton X-100-insoluble material and lipopolysaccharide and phospholipids. Liposomes reconstituted from lipopolysaccharide and phospholipids were impermeable to sucrose (Mr, 342) and to a high-molecular-weight dextran (average Mr, 6,600). When the Triton X-100-insoluble material was introduced into the reconstituted liposomes, the vesicles became permeable to sucrose, raffinose (Mr, 504), and stachyose (Mr, 666) and fully retained dextrans of Mr greater than 1,500. Inulin (average Mr, 1,400) was tested for its efflux from the reconstituted outer membrane vesicles; 62% of the added inulin was trapped. The molecular weight exclusion limit for the outer membrane of H. influenzae type b was therefore estimated at approximately 1,400. A protein responsible for the transmembrane diffusion of solutes was purified from H. influenzae type b by extraction of whole cells with cetyl trimethyl ammonium bromide. When this extract was passed over DEAE-Sepharose, three protein-containing peaks (I, II, and III) were eluted. Peaks I and II contained mixtures of proteins as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis; when tested for their pore-forming properties, these proteins were unable to render liposomes of lipopolysaccharide and phospholipid permeable to sucrose. Peak III contained only one molecular species of protein of molecular weight 40,000; this protein acted as a porin in reconstituted vesicles. The molecular weight exclusion limit for 40,000-molecular-weight protein matched the estimate of approximately 1,400 which was determined for outer membranes. A series of homologous saccharides of increasing degree of polymerization was prepared from agarose by hydrolysis with beta-agarase and fractionation on gel filtration chromatography. These oligosaccharides of Mr, 936, 1,242, 1,548, and 1,854 were assayed for retention by the complete vesicles containing 40-kilodalton protein and lipopolysaccharide and phospholipids. All of these oligosaccharides were lost by efflux through the porin. Since the molecular conformation of the largest oligosaccharide is an elongated semirigid helix, it is suggested that the pore formed by the 40-kilodalton protein does not act as a barrier to the diffusion of this compound.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Angus B. L., Carey A. M., Caron D. A., Kropinski A. M., Hancock R. E. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-supersusceptible mutant. Antimicrob Agents Chemother. 1982 Feb;21(2):299–309. doi: 10.1128/aac.21.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S., Fulmer A., Scott W. E., Dea I. C., Moorhouse R., Rees D. A. The agarose double helix and its function in agarose gel structure. J Mol Biol. 1974 Dec 5;90(2):269–284. doi: 10.1016/0022-2836(74)90372-6. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C. Purification and partial characterization of the major outer membrane protein of Neisseria gonorrhoeae. Infect Immun. 1982 Apr;36(1):277–283. doi: 10.1128/iai.36.1.277-283.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Coulton J. W., Mason P., Dorrance D. The permeability barrier of Haemophilus influenzae type b against beta-lactam antibiotics. J Antimicrob Chemother. 1983 Nov;12(5):435–449. doi: 10.1093/jac/12.5.435. [DOI] [PubMed] [Google Scholar]
- Coulton J. W., Wan D. T. The outer membrane of haemophilus influenzae type b: cell envelope associations of major proteins. Can J Microbiol. 1983 Feb;29(2):280–287. doi: 10.1139/m83-046. [DOI] [PubMed] [Google Scholar]
- Decad G. M., Nikaido H. Outer membrane of gram-negative bacteria. XII. Molecular-sieving function of cell wall. J Bacteriol. 1976 Oct;128(1):325–336. doi: 10.1128/jb.128.1.325-336.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Decad G. M., Nikaido H. Identification of the protein producing transmembrane diffusion pores in the outer membrane of Pseudomonas aeruginosa PA01. Biochim Biophys Acta. 1979 Jul 5;554(2):323–331. doi: 10.1016/0005-2736(79)90373-0. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson T. G., Polson A. Some physical characteristics of the agarose molecule. Biochim Biophys Acta. 1968 Aug 6;165(1):43–58. doi: 10.1016/0304-4165(68)90186-4. [DOI] [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Janda J., Work E. A colorimetric estimation of lipopolysaccharides. FEBS Lett. 1971 Sep 1;16(4):343–345. doi: 10.1016/0014-5793(71)80386-1. [DOI] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976 Jan;22(1):29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella typhimurium: reconstitution of sucrose-permeable membrane vesicles. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1224–1230. doi: 10.1016/0006-291x(75)90823-2. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Nixdorff K., Fitzer H., Gmeiner J., Martin H. H. Reconstitution of model membranes from phospholipid and outer membrane proteins of Proteus mirabilis. Role of proteins in the formation of hydrophilic pores and protection of membranes against detergents. Eur J Biochem. 1977 Nov 15;81(1):63–69. doi: 10.1111/j.1432-1033.1977.tb11927.x. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Shuman H. A. The maltose-maltodextrin transport system of Escherichia coli. Ann Microbiol (Paris) 1982 Jan;133A(1):153–159. [PubMed] [Google Scholar]
- Tokunaga H., Tokunaga M., Nakae T. Characterization of porins from the outer membrane of Salmonella typhimurium. 1. Chemical analysis. Eur J Biochem. 1979 Apr;95(3):433–439. doi: 10.1111/j.1432-1033.1979.tb12982.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Permeability of Pseudomonas aeruginosa outer membrane to hydrophilic solutes. J Bacteriol. 1982 Nov;152(2):636–642. doi: 10.1128/jb.152.2.636-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]