Abstract
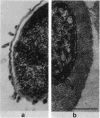
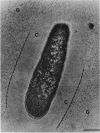
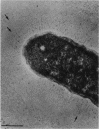
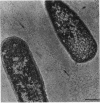
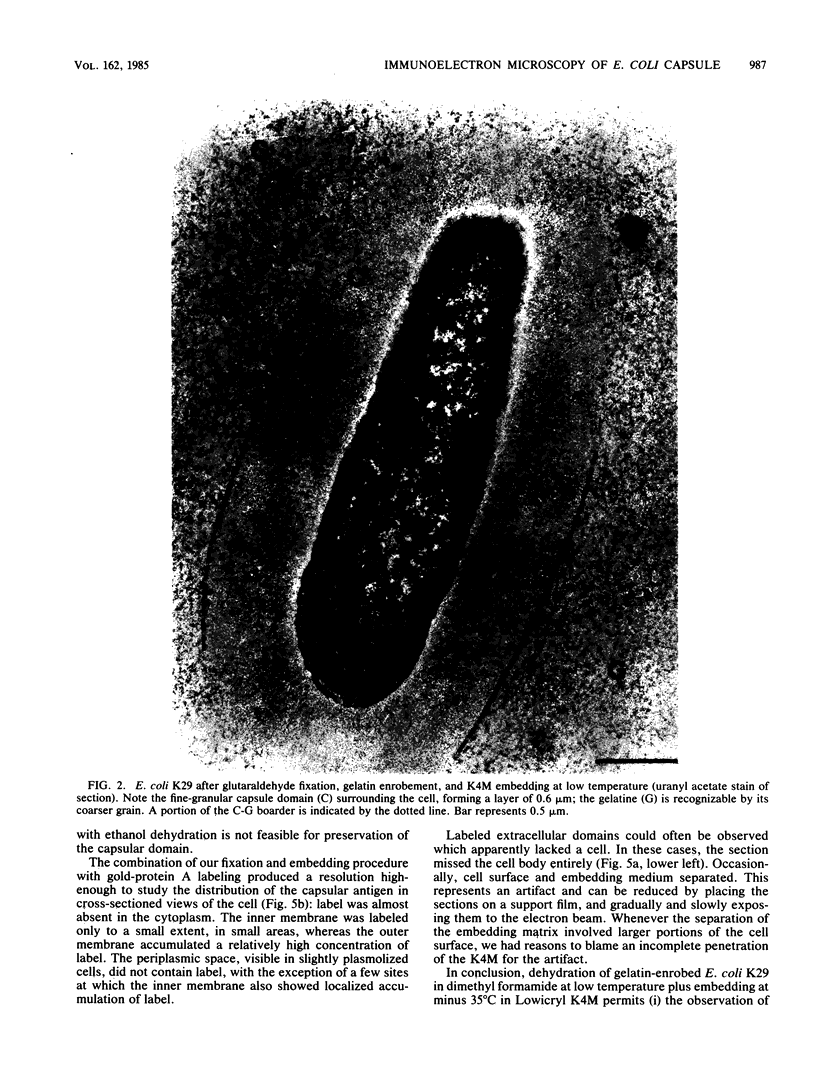
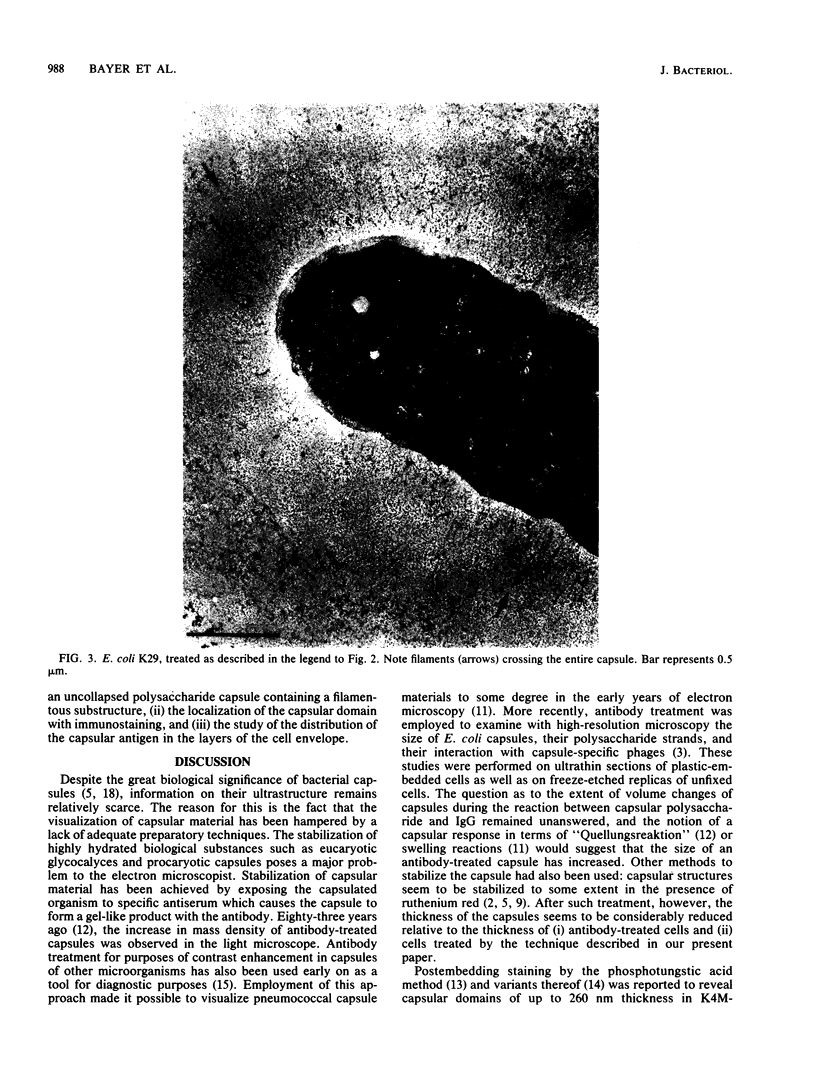
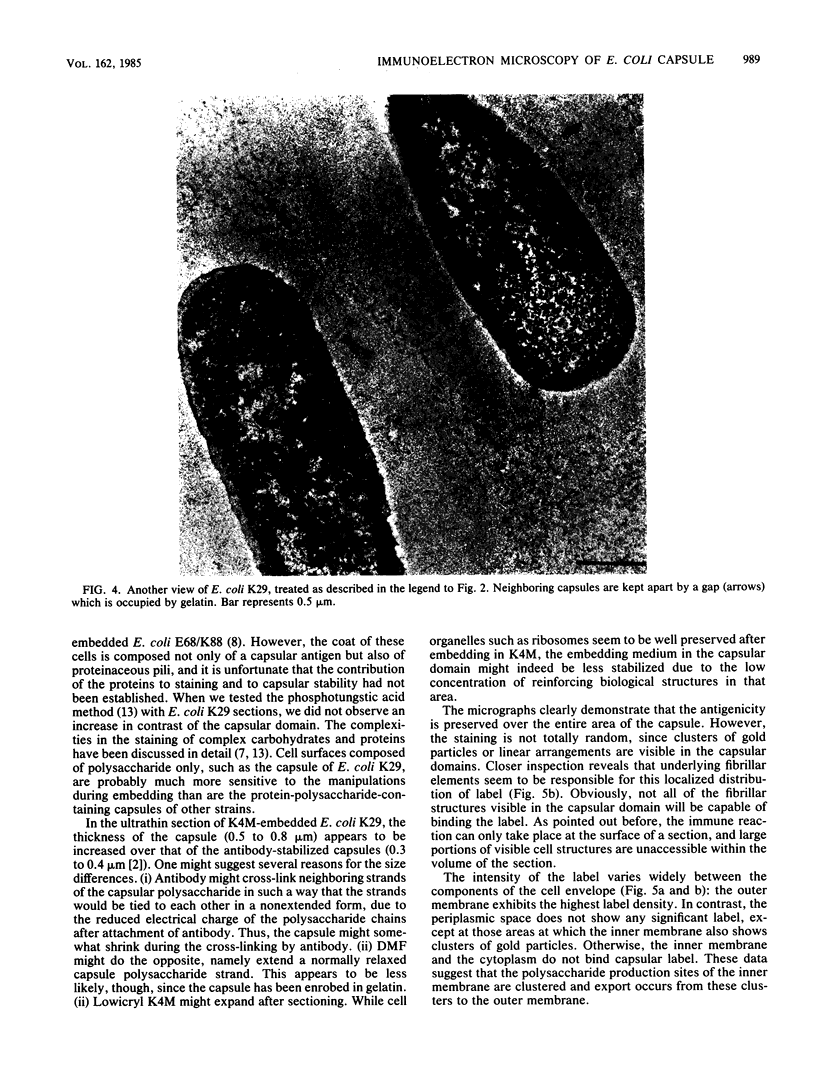
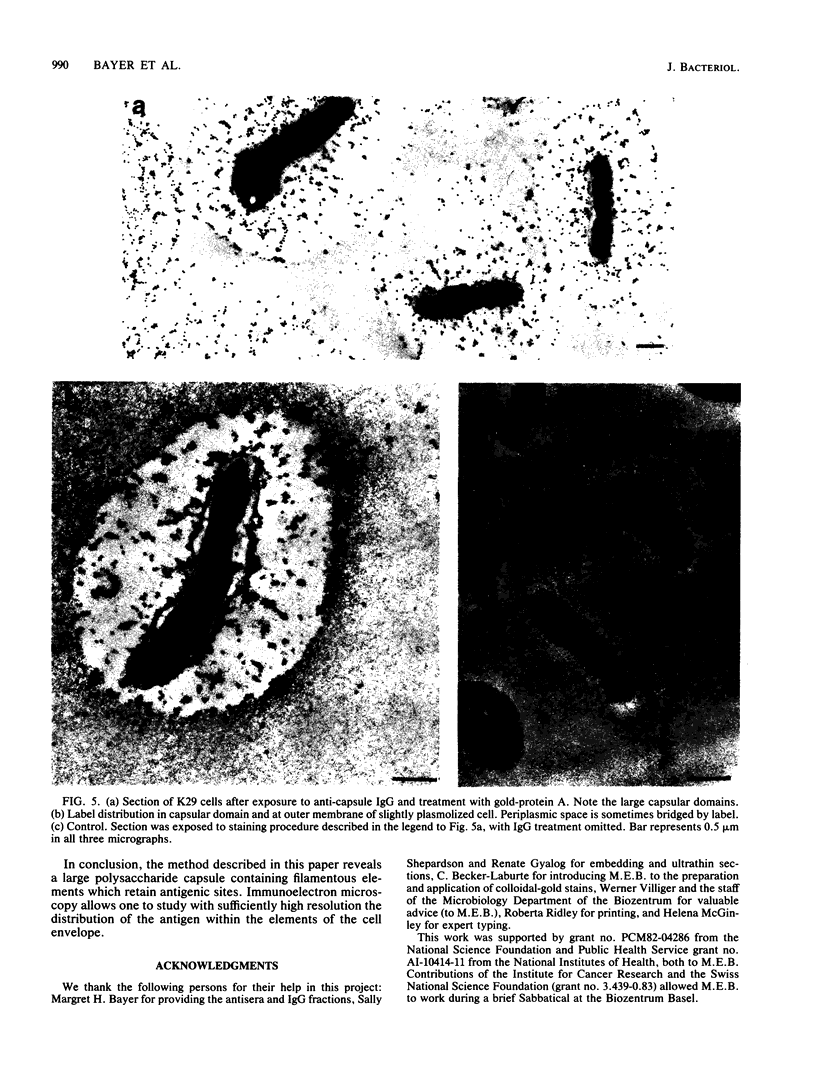
The polysaccharide capsule of Escherichia coli K29 fully surrounds the microorganism and thus occupies an extracellular space ca. 20 times larger in volume than that of the decapsulated cell. Since more than 95% of the capsule consists of water, dehydration for electron microscopy causes the material to collapse. We describe here a method for embedding the capsule in an uncollapsed form. Dehydration of gelatin-enrobed, glutaraldehyde-fixed cells was performed in dimethyl formamide. The cells were embedded in Lowicryl K4M with the "progressive lowering of temperature" method and UV polymerization. In ultrathin sections, the capsule can be identified by its low electron contrast. It occupies a layer 3/4 micron thick thick and shows fibrous strands embedded in a fine granular matrix. The thin strands extend radially from the cell wall and transverse the capsule. The entire capsule domain, as well as the outer membrane, binds specific anticapsular antibody, whereas the periplasmic space and most of the inner membrane lack capsule-specific immunostain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armbruster B. L., Carlemalm E., Chiovetti R., Garavito R. M., Hobot J. A., Kellenberger E., Villiger W. Specimen preparation for electron microscopy using low temperature embedding resins. J Microsc. 1982 Apr;126(Pt 1):77–85. doi: 10.1111/j.1365-2818.1982.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Thurow H., Bayer M. H. Penetration of the polysaccharide capsule of Escherichia coli (Bi161/42) by bacteriophage K29. Virology. 1979 Apr 15;94(1):95–118. doi: 10.1016/0042-6822(79)90441-0. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol. 1977 May;130(2):911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- Fehmel F., Feige U., Niemann H., Stirm S. Escherichia coli capsule bacteriophages. VII. Bacteriophage 29-host capsular polysaccharide interactions. J Virol. 1975 Sep;16(3):591–601. doi: 10.1128/jvi.16.3.591-601.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick D., Scott J. E. Phosphotungstic acid not a stain for polysaccharide. J Histochem Cytochem. 1970 Jun;18(6):455–455. doi: 10.1177/18.6.455. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Moorhouse R., Winter W. T., Arnott S., Bayer M. E. Conformation and molecular organization in fibers of the capsular polysaccharide from Escherichia coli M41 mutant. J Mol Biol. 1977 Jan 25;109(3):373–391. doi: 10.1016/s0022-2836(77)80018-1. [DOI] [PubMed] [Google Scholar]
- Pease D. C. Phosphotungstic acid as a specific electron stain for complex carbohydrates. J Histochem Cytochem. 1970 Jun;18(6):455–458. doi: 10.1177/18.6.455-a. [DOI] [PubMed] [Google Scholar]
- Springer E. L., Roth I. L. The ultrastructure of the capsules of Diplococcus pneumoniae and Klebsiella pneumoniae stained with ruthenium red. J Gen Microbiol. 1973 Jan;74(1):21–31. doi: 10.1099/00221287-74-1-21. [DOI] [PubMed] [Google Scholar]
- Stirm S., Freund-Mölbert E. Escherichia coli capsule bacteriophages. II. Morphology. J Virol. 1971 Sep;8(3):330–342. doi: 10.1128/jvi.8.3.330-342.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]