Abstract
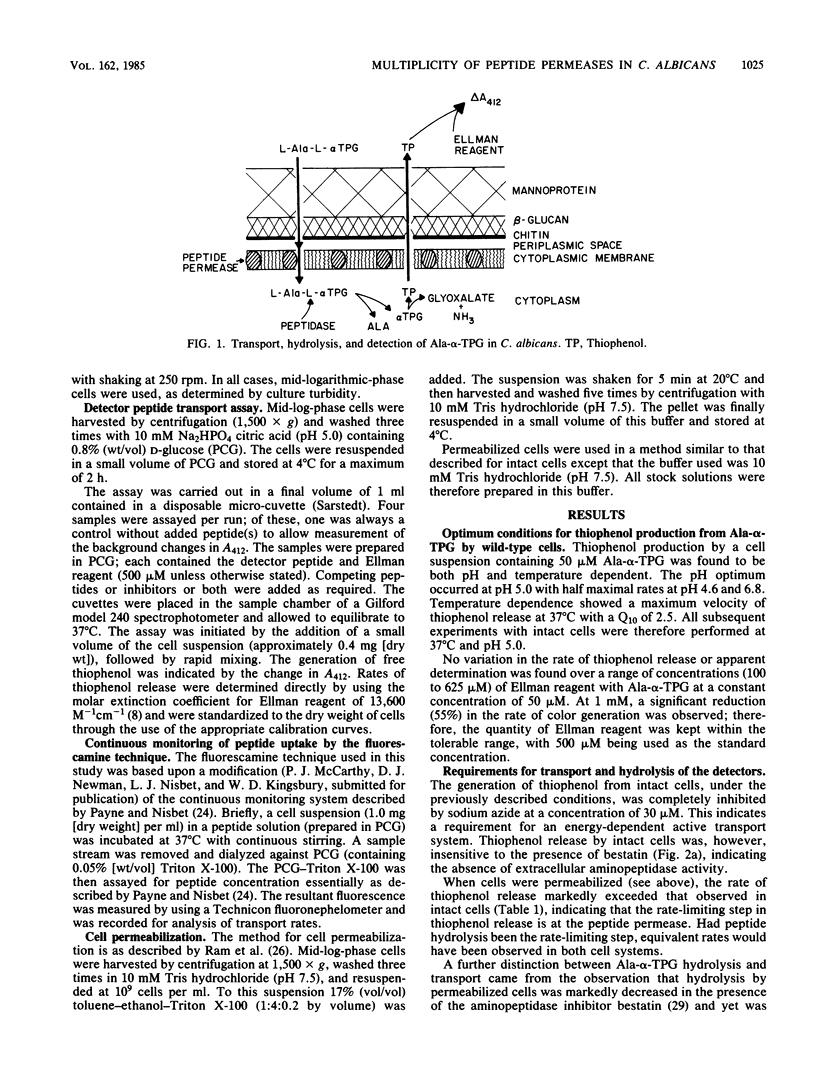
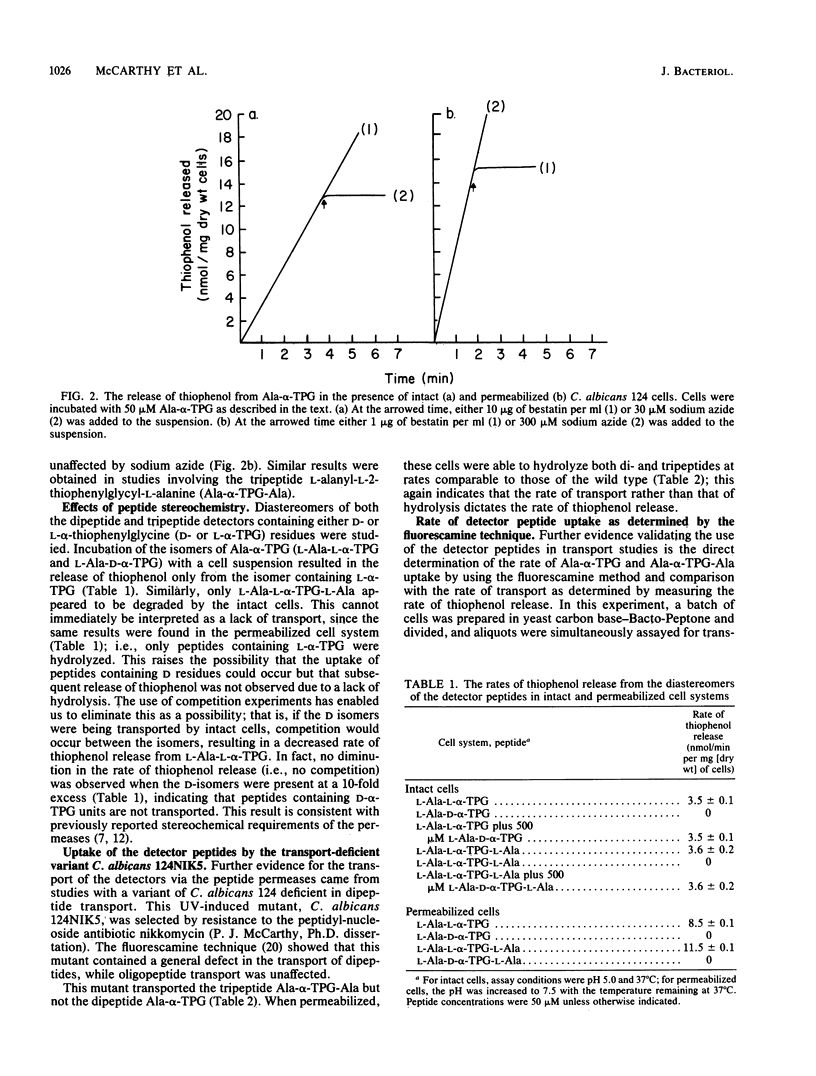
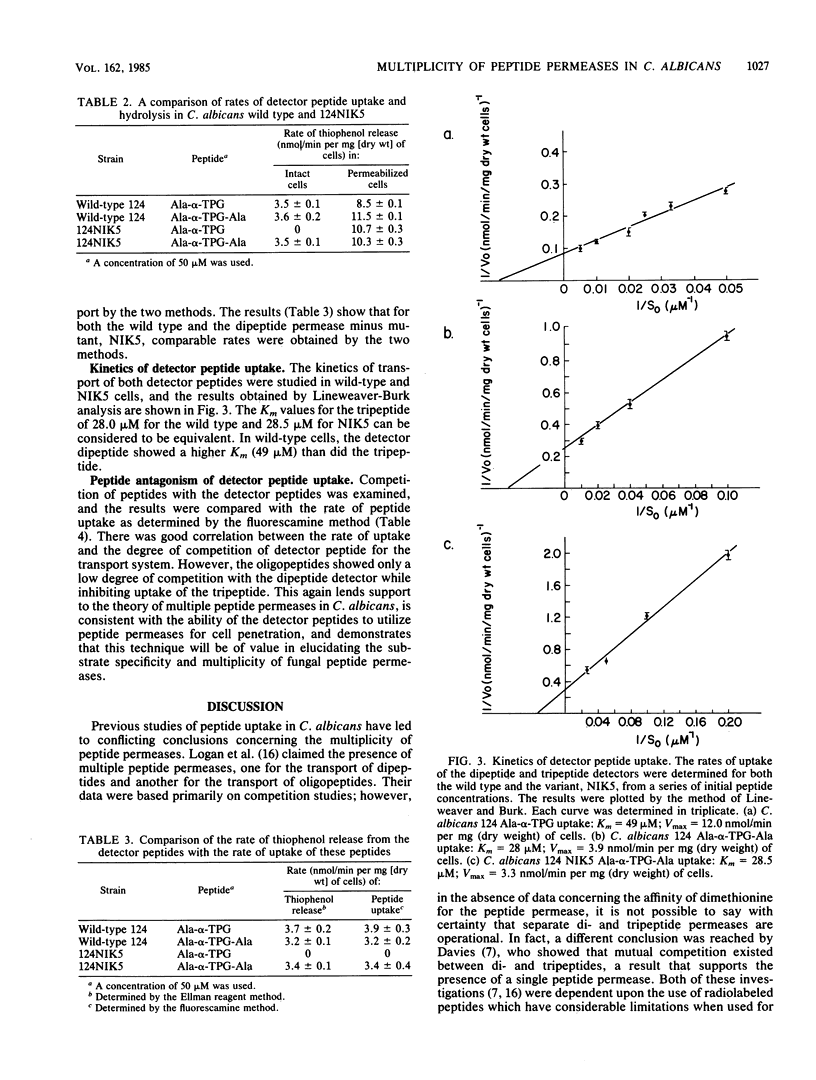
Evidence is presented for the presence of multiple peptide permeases in the eucaryotic organism Candida albicans. Instrumental in these studies were the peptides L-alanyl-L-2-thiophenylglycine (Ala-alpha-TPG) and L-alanyl-L-2-thiophenylglycyl-L-alanine (Ala-alpha-TPG-Ala), which contain thiophenol attached to the alpha-carbon of glycine. Subsequent to transport into the fungal cell, enzymatic hydrolysis of these peptides resulted in the release of free thiophenol, which was quantified by using Ellman reagent. Thiophenol release was shown to be directly correlated to peptide transport and hydrolysis, with transport being the rate-limiting step in intact cells. These peptides, whose uptake showed Michaelis-Menten kinetics, have been used to determine peptide uptake in C. albicans. In addition, we found that the intracellular peptidases can readily be assayed in permeabilized cells and that bestatin, an aminopeptidase inhibitor, inhibits all detectable peptidase activity. C. albicans 124 was able to transport and hydrolyze both Ala-alpha-TPG and Ala-alpha-TPG-Ala, whereas the mutant (124NIK5) was able to transport only the tripeptide. The intracellular peptidases of this mutant were unaffected. In wild-type C. albicans 124, oligopeptides were able to compete with uptake of Ala-alpha-TPG-Ala to a far greater extent than with that of Ala-alpha-TPG; dipeptides inhibited uptake of both Ala-alpha-TPG and Ala-alpha-TPG-Ala. These results provide complementary evidence for the existence of distinct transport systems.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. G., Atherton F. R., Hall M. J., Hassall C. H., Holmes S. W., Lambert R. W., Nisbet L. J., Ringrose P. S. Phosphonopeptides, a new class of synthetic antibacterial agents. Nature. 1978 Mar 2;272(5648):56–58. doi: 10.1038/272056a0. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Ames G. F., Young J. D., Tsuchiya D., Lecocq J. Illicit transport: the oligopeptide permease. Proc Natl Acad Sci U S A. 1973 Feb;70(2):456–458. doi: 10.1073/pnas.70.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekcer J. M., Naider F. Peptide transport in yeast: uptake of radioactive trimethionine in Saccharomyces cerevisiae. Arch Biochem Biophys. 1977 Jan 15;178(1):245–255. doi: 10.1016/0003-9861(77)90189-8. [DOI] [PubMed] [Google Scholar]
- Davies M. B. Peptide uptake in Candida albicans. J Gen Microbiol. 1980 Nov;121(1):181–186. doi: 10.1099/00221287-121-1-181. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fickel T. E., Gilvarg C. Transport of impermeant substances in E. coli by way of oligopeptide permease. Nat New Biol. 1973 Feb 7;241(110):161–163. doi: 10.1038/newbio241161a0. [DOI] [PubMed] [Google Scholar]
- Iwata K. Fungal toxins as a parasitic factor responsible for the establishment of fungal infections. Mycopathologia. 1978 Dec 18;65(1-3):141–154. doi: 10.1007/BF00447185. [DOI] [PubMed] [Google Scholar]
- Kenig M., Abraham E. P. Antimicrobial activities and antagonists of bacilysin and anticapsin. J Gen Microbiol. 1976 May;94(1):37–45. doi: 10.1099/00221287-94-1-37. [DOI] [PubMed] [Google Scholar]
- Kingsbury W. D., Boehm J. C., Mehta R. J., Grappel S. F., Gilvarg C. A novel peptide delivery system involving peptidase activated prodrugs as antimicrobial agents. Synthesis and biological activity of peptidyl derivatives of 5-fluorouracil. J Med Chem. 1984 Nov;27(11):1447–1451. doi: 10.1021/jm00377a012. [DOI] [PubMed] [Google Scholar]
- Kingsbury W. D., Boehm J. C., Mehta R. J., Grappel S. F. Transport of antimicrobial agents using peptide carrier systems: anticandidal activity of m-fluorophenylalanine--peptide conjugates. J Med Chem. 1983 Dec;26(12):1725–1729. doi: 10.1021/jm00366a013. [DOI] [PubMed] [Google Scholar]
- Kingsbury W. D., Boehm J. C., Perry D., Gilvarg C. Portage of various compounds into bacteria by attachment to glycine residues in peptides. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4573–4576. doi: 10.1073/pnas.81.14.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichliter W. D., Naider F., Becker J. M. Basis for the design of anticandidal agents from studies of peptide utilization in Canadida albicans. Antimicrob Agents Chemother. 1976 Sep;10(3):483–490. doi: 10.1128/aac.10.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan D. A., Becker J. M., Naider F. Peptide transport in Candida albicans. J Gen Microbiol. 1979 Sep;114(1):179–186. doi: 10.1099/00221287-114-1-179. [DOI] [PubMed] [Google Scholar]
- Marder R., Rose B., Becker J. M., Naider F. Isolation of a peptide transport-deficient mutant of yeast. J Bacteriol. 1978 Dec;136(3):1174–1177. doi: 10.1128/jb.136.3.1174-1177.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R. J., Kingsbury W. D., Valenta J., Actor P. Anti-Candida activity of polyoxin: example of peptide transport in yeasts. Antimicrob Agents Chemother. 1984 Mar;25(3):373–374. doi: 10.1128/aac.25.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naider F., Shenbagamurthi P., Steinfeld A. S., Smith H. A., Boney C., Becker J. M. Synthesis and biological activity of tripeptidyl polyoxins as antifungal agents. Antimicrob Agents Chemother. 1983 Nov;24(5):787–796. doi: 10.1128/aac.24.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J. W., Nisbet T. M. Limitations to the use of radioactively labelled substrates for studying peptide transport in microorganisms. FEBS Lett. 1980 Sep 22;119(1):73–76. doi: 10.1016/0014-5793(80)81000-3. [DOI] [PubMed] [Google Scholar]
- Perry D., Gilvarg C. Spectrophotometric determination of affinities of peptides for their transport systems in Escherichia coli. J Bacteriol. 1984 Dec;160(3):943–948. doi: 10.1128/jb.160.3.943-948.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram S. P., Sullivan P. A., Shepherd M. G. The in situ assay of Candida albicans enzymes during yeast growth and germ-tube formation. J Gen Microbiol. 1983 Aug;129(8):2367–2378. doi: 10.1099/00221287-129-8-2367. [DOI] [PubMed] [Google Scholar]
- Ti J. S., Steinfeld A. S., Naider F., Gulumoglu A., Lewis S. V., Becker J. M. Anticandidal activity of pyrimidine-peptide conjugates. J Med Chem. 1980 Aug;23(8):913–918. doi: 10.1021/jm00182a019. [DOI] [PubMed] [Google Scholar]
- Ugolev A. M., Timofeeva N. M., Smirnova L. F., Delaey P., Gruzdkov A. A., Iezuitova N. N., Mityushova N. M., Roshchina G. M., Gurman E. G., Gusev V. M. Membrane and intracellular hydrolysis of peptides: differentiation, role and interrelations with transport. Ciba Found Symp. 1977;(50):221–243. doi: 10.1002/9780470720318.ch13. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Aoyagi T., Suda H., Hamada M., Takeuchi T. Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes. J Antibiot (Tokyo) 1976 Jan;29(1):97–99. doi: 10.7164/antibiotics.29.97. [DOI] [PubMed] [Google Scholar]
- Wolfinbarger L., Jr, Marzluf G. A. Specificity and regulation of peptide transport on Neurospora crassa. Arch Biochem Biophys. 1975 Dec;171(2):637–644. doi: 10.1016/0003-9861(75)90074-0. [DOI] [PubMed] [Google Scholar]


