Abstract
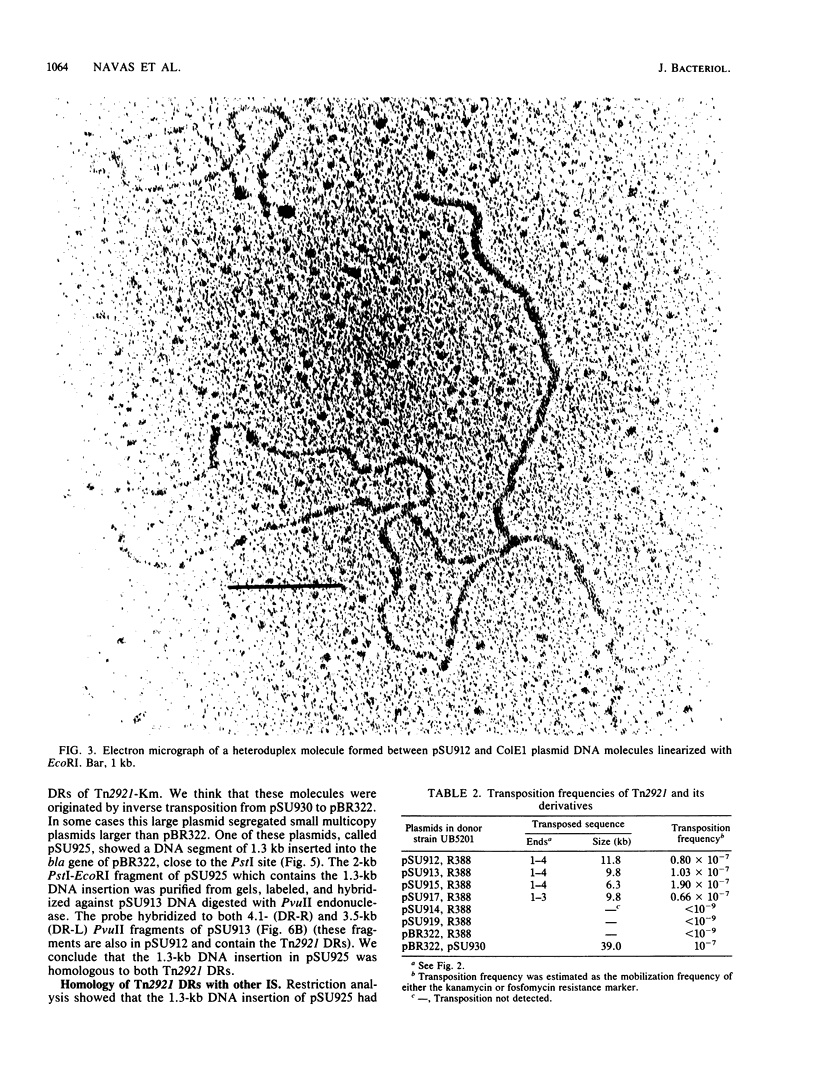
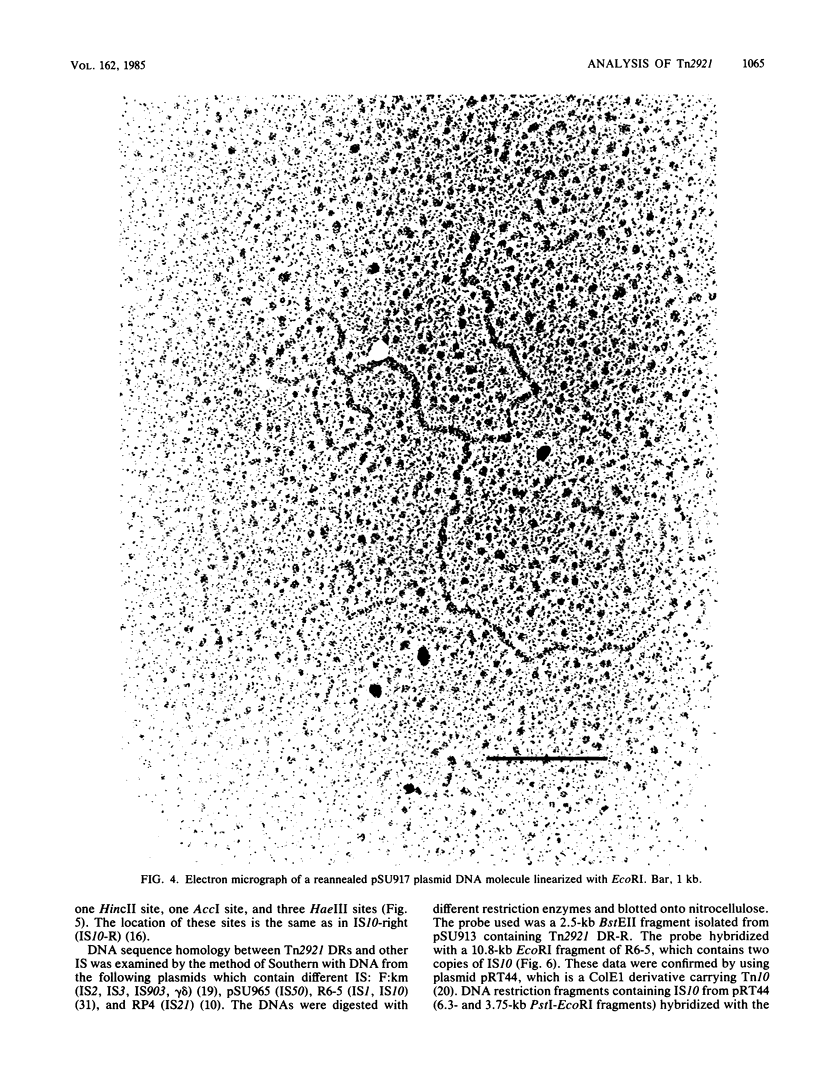
The fosfomycin resistance transposon Tn2921 is flanked by directly repeated sequences homologous to the Tn10-related insertion sequence IS10. The nonrepeated DNA sequences of Tn2921 can be deleted without affecting the transposition ability of the element, showing that at least one of the direct repeats is an active insertion sequence. Transposition of Tn2921 seems to occur through direct transposition, since cointegrates have not been observed. The evolutionary relatedness of Tn2921 and IS10 is discussed.
Full text
PDF
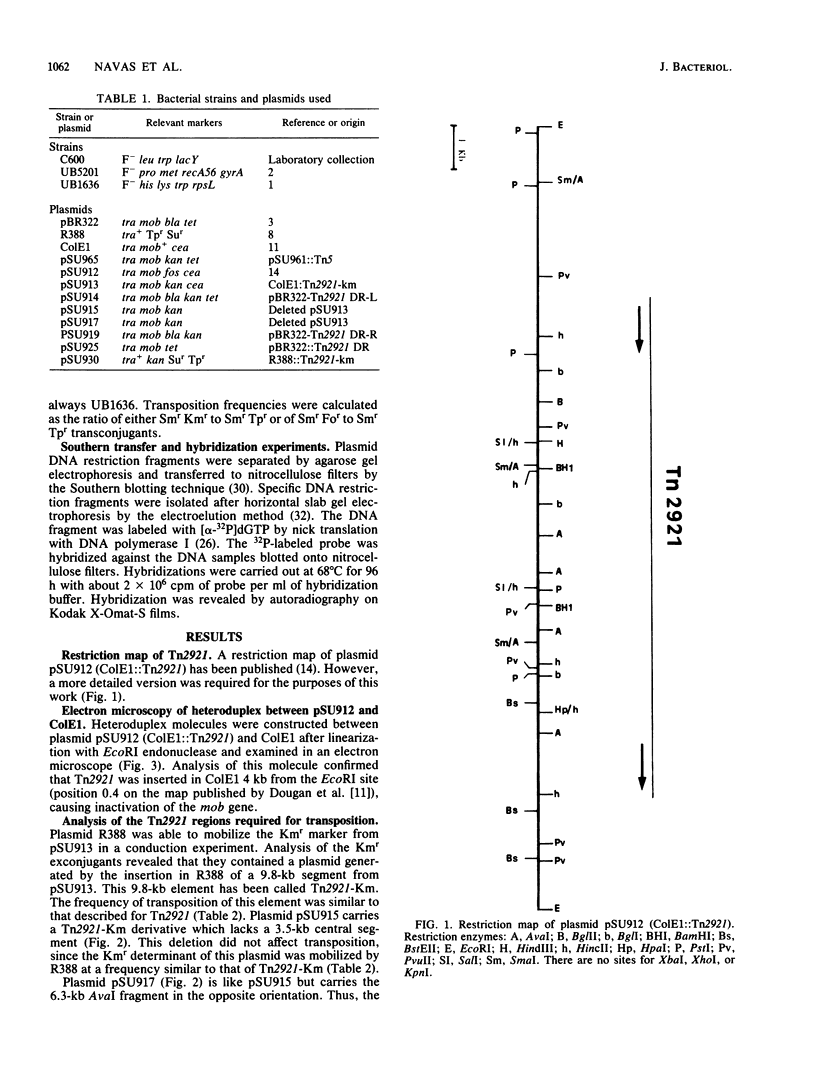
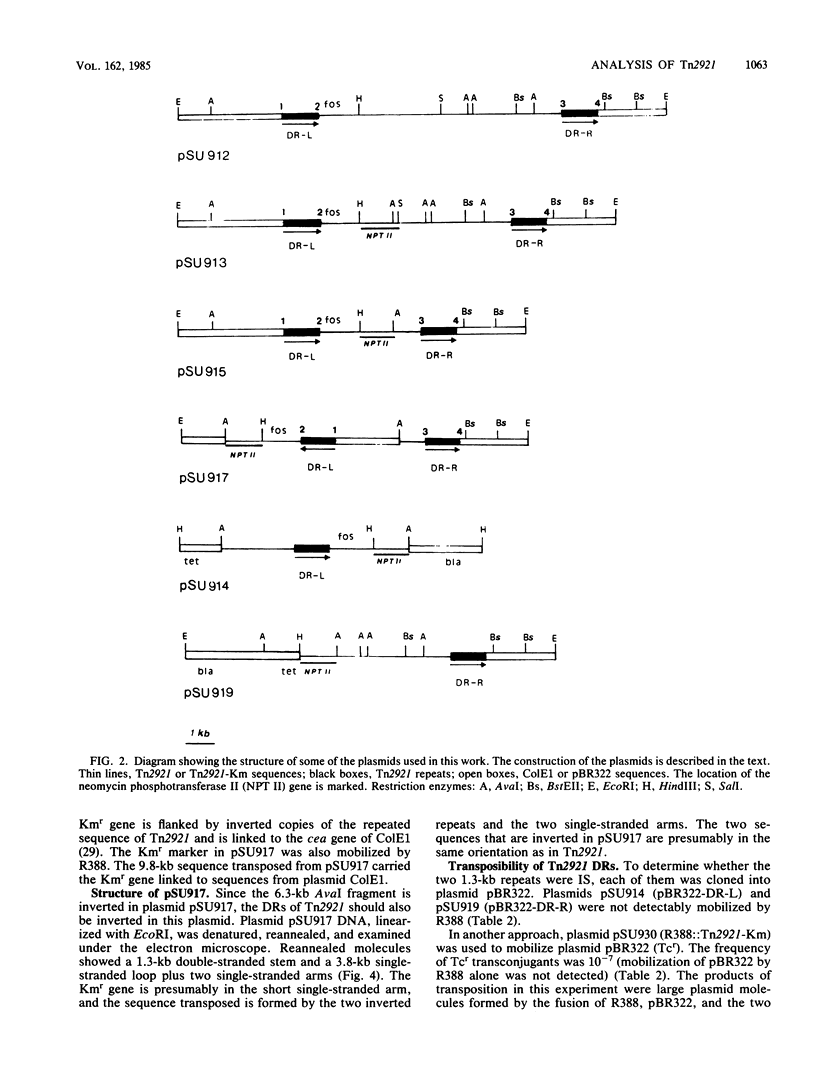


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P. M., Richmond M. H. Translocation of a discrete piece of deoxyribonucleic acid carrying an amp gene between replicons in Eschericha coli. J Bacteriol. 1976 Apr;126(1):1–6. doi: 10.1128/jb.126.1.1-6.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Clark A. J., Warren G. J. Conjugal transmission of plasmids. Annu Rev Genet. 1979;13:99–125. doi: 10.1146/annurev.ge.13.120179.000531. [DOI] [PubMed] [Google Scholar]
- Clerget M., Chandler M., Caro L. Isolation of an IS1 flanked kanamycin resistance transposon from R1drd19. Mol Gen Genet. 1980;180(1):123–127. doi: 10.1007/BF00267360. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Trimethoprim resistance conferred by W plasmids in Enterobacteriaceae. J Gen Microbiol. 1972 Sep;72(2):349–355. doi: 10.1099/00221287-72-2-349. [DOI] [PubMed] [Google Scholar]
- Depicker A., De Block M., Inzé D., Van Montagu M., Schell J. IS-like element IS8 in RP4 plasmid and its involvement in cointegration. Gene. 1980 Sep;10(4):329–338. doi: 10.1016/0378-1119(80)90153-5. [DOI] [PubMed] [Google Scholar]
- Foster T. J., Lundblad V., Hanley-Way S., Halling S. M., Kleckner N. Three Tn10-associated excision events: relationship to transposition and role of direct and inverted repeats. Cell. 1981 Jan;23(1):215–227. doi: 10.1016/0092-8674(81)90286-5. [DOI] [PubMed] [Google Scholar]
- García-Lobo J. M., León J., Navas J., Ortiz J. M. Cloning and expression in minicells of the determinant of resistance to fosfomycin from the transposon Tn2921. Plasmid. 1984 May;11(3):243–247. doi: 10.1016/0147-619x(84)90030-1. [DOI] [PubMed] [Google Scholar]
- García-Lobo J. M., Ortiz J. M. Tn292l, a transposon encoding fosfomycin resistance. J Bacteriol. 1982 Jul;151(1):477–479. doi: 10.1128/jb.151.1.477-479.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted J., de la Cruz F., Altenbuchner J., Schmitt R. Complementation of transposition of tnpA mutants of Tn3, Tn21, Tn501, and Tn1721. Plasmid. 1982 Nov;8(3):276–286. doi: 10.1016/0147-619x(82)90065-8. [DOI] [PubMed] [Google Scholar]
- Halling S. M., Simons R. W., Way J. C., Walsh R. B., Kleckner N. DNA sequence organization of IS10-right of Tn10 and comparison with IS10-left. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2608–2612. doi: 10.1073/pnas.79.8.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harayama S., Oguchi T., Iino T. Does Tn10 transpose via the cointegrate molecule? Mol Gen Genet. 1984;194(3):444–450. doi: 10.1007/BF00425556. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Berg D. E., Allet B., Reznikoff W. S. Restriction enzyme cleavage map of Tn10, a transposon which encodes tetracycline resistance. J Bacteriol. 1979 Jan;137(1):681–685. doi: 10.1128/jb.137.1.681-685.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- Mendoza C., Garcia J. M., Llaneza J., Mendez F. J., Hardisson C., Ortiz J. M. Plasmid-determined resistance to fosfomycin in Serratia marcescens. Antimicrob Agents Chemother. 1980 Aug;18(2):215–219. doi: 10.1128/aac.18.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ross D. G., Grisafi P., Kleckner N., Botstein D. The ends of Tn10 are not IS3. J Bacteriol. 1979 Sep;139(3):1097–1101. doi: 10.1128/jb.139.3.1097-1101.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D. G., Swan J., Kleckner N. Physical structures of Tn10-promoted deletions and inversions: role of 1400 bp inverted repetitions. Cell. 1979 Apr;16(4):721–731. doi: 10.1016/0092-8674(79)90088-6. [DOI] [PubMed] [Google Scholar]
- Sabik J. F., Suit J. L., Luria S. E. cea-kil operon of the ColE1 plasmid. J Bacteriol. 1983 Mar;153(3):1479–1485. doi: 10.1128/jb.153.3.1479-1485.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]