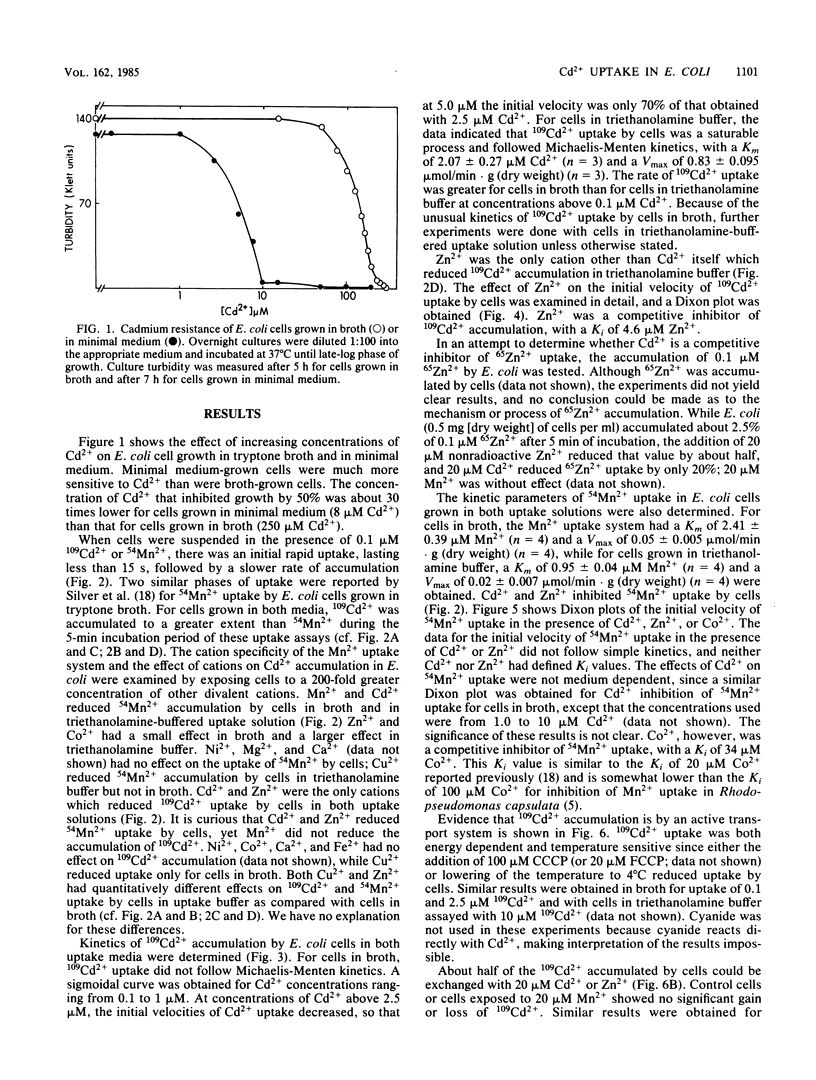
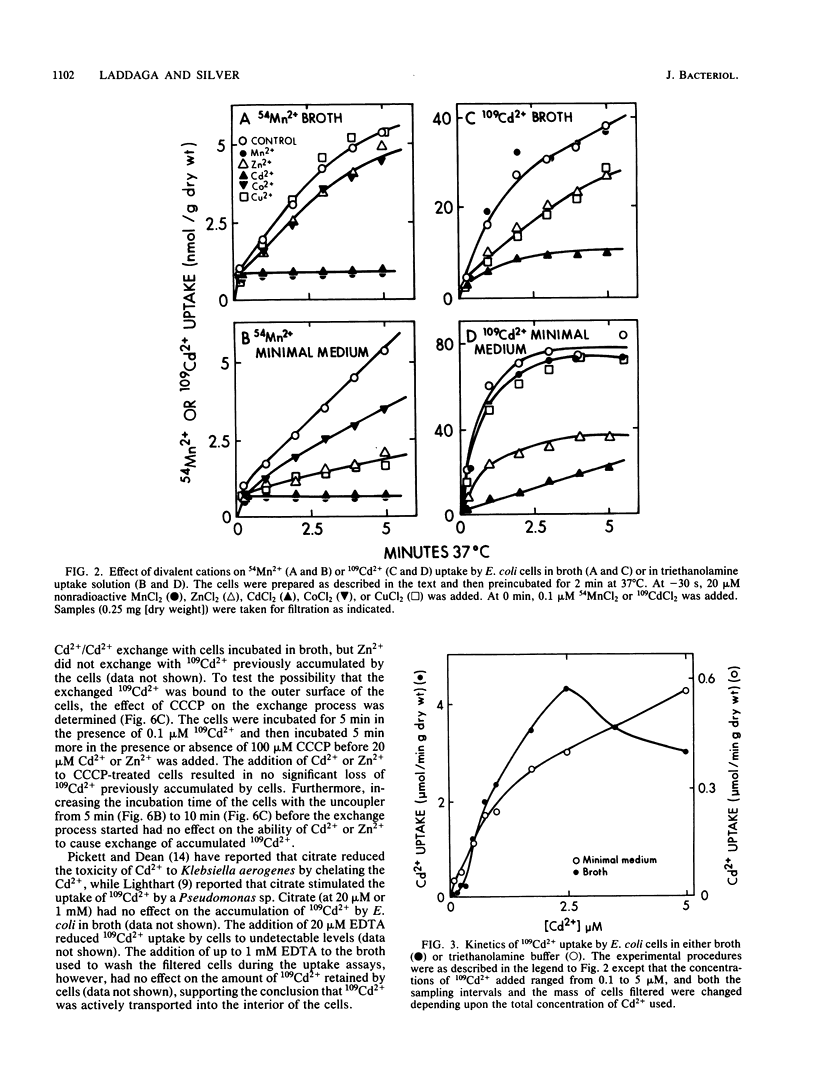
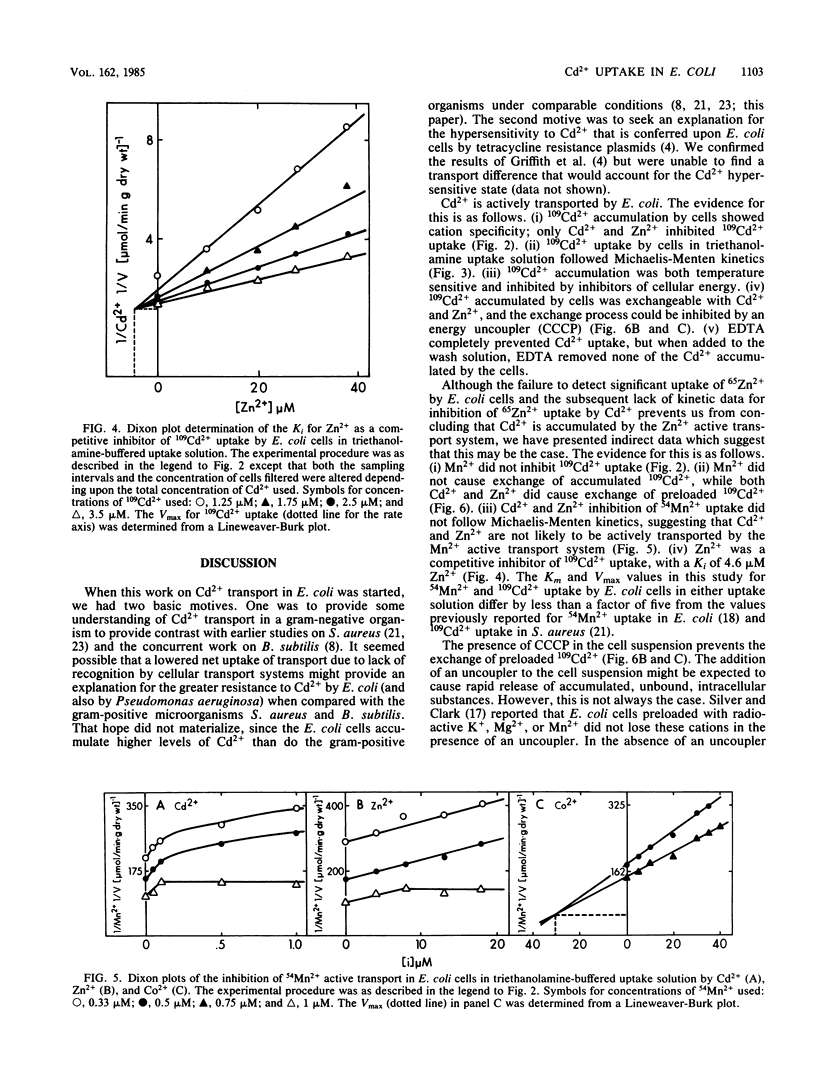
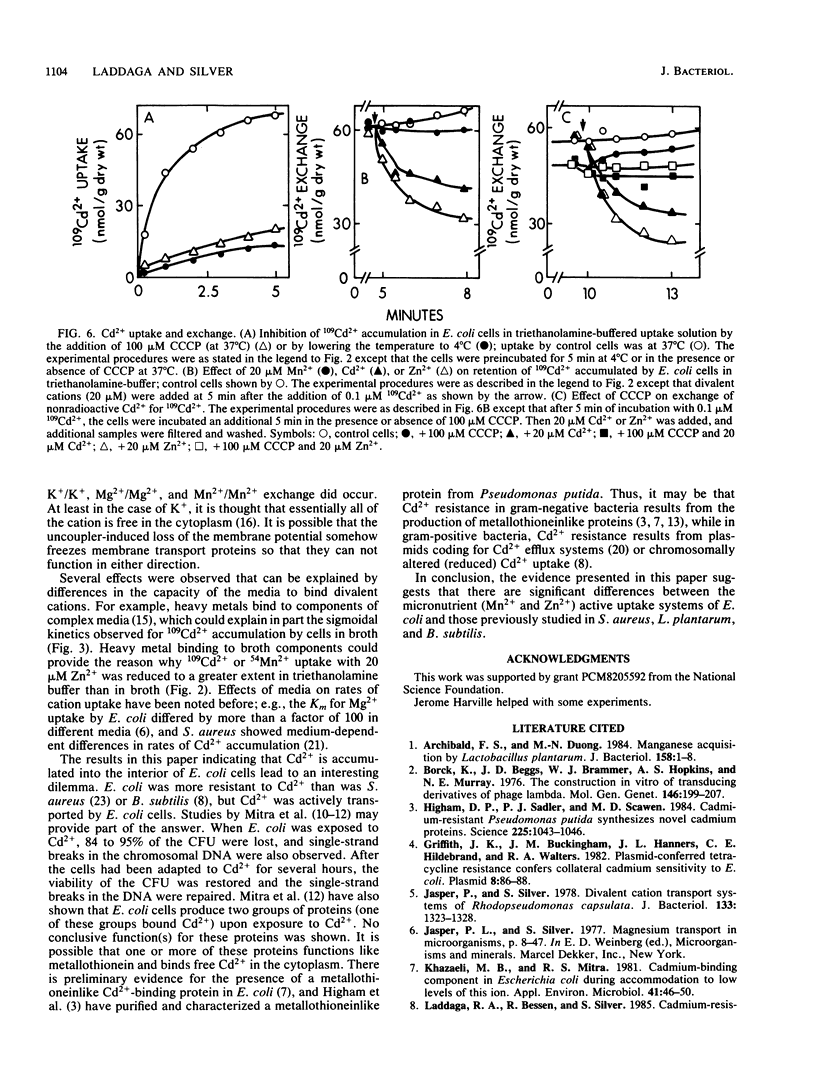
Abstract
109Cd2+ uptake by Escherichia coli occurred by means of an active transport system which has a Km of 2.1 microM Cd2+ and a Vmax of 0.83 mumol/min X g (dry weight) in uptake buffer. 109Cd2+ accumulation was both energy dependent and temperature sensitive. The addition of 20 microM Cd2+ or Zn2+ (but not Mn2+) to the cell suspensions preloaded with 109Cd2+ caused the exchange of Cd2+. 109Cd2+ (0.1 microM) uptake by cells was inhibited by the addition of 20 microM Zn2+ but not Mn2+. Zn2+ was a competitive inhibitor of 109Cd2+ uptake with an apparent Ki of 4.6 microM Zn2+. Although Mn2+ did not inhibit 109Cd2+ uptake, the addition of either 20 microM Cd2+ or Zn2+ prevented the uptake of 0.1 microM 54Mn2+, which apparently occurs by a separate transport system. The inhibition of 54Mn2+ accumulation by Cd2+ or Zn2+ did not follow Michaelis-Menten kinetics and had no defined Ki values. Co2+ was a competitive inhibitor of Mn2+ uptake with an apparent Ki of 34 microM Co2+. We were unable to demonstrate an active transport system for 65Zn2+ in E. coli.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S., Duong M. N. Manganese acquisition by Lactobacillus plantarum. J Bacteriol. 1984 Apr;158(1):1–8. doi: 10.1128/jb.158.1.1-8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Griffith J. K., Buckingham J. M., Hanners J. L., Hildebrand C. E., Walters R. A. Plasmid-conferred tetracycline resistance confers collateral cadmium sensitivity of E. coli cells. Plasmid. 1982 Jul;8(1):86–88. doi: 10.1016/0147-619x(82)90044-0. [DOI] [PubMed] [Google Scholar]
- Higham D. P., Sadler P. J., Scawen M. D. Cadmium-Resistant Pseudomonas putida Synthesizes Novel Cadmium Proteins. Science. 1984 Sep 7;225(4666):1043–1046. doi: 10.1126/science.225.4666.1043. [DOI] [PubMed] [Google Scholar]
- Jasper P., Silver S. Divalent cation transport systems of Rhodopseudomonas capsulata. J Bacteriol. 1978 Mar;133(3):1323–1328. doi: 10.1128/jb.133.3.1323-1328.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaeli M. B., Mitra R. S. Cadmium-binding component in Escherichia coli during accommodation to low levels of this ion. Appl Environ Microbiol. 1981 Jan;41(1):46–50. doi: 10.1128/aem.41.1.46-50.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laddaga R. A., Bessen R., Silver S. Cadmium-resistant mutant of Bacillus subtilis 168 with reduced cadmium transport. J Bacteriol. 1985 Jun;162(3):1106–1110. doi: 10.1128/jb.162.3.1106-1110.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthart B. Effects of certain cadmium species on pure and litter populations of microorganisms. Antonie Van Leeuwenhoek. 1980;46(2):161–167. doi: 10.1007/BF00444071. [DOI] [PubMed] [Google Scholar]
- Mitra R. S., Bernstein I. A. Single-strand breakage in DNA of Escherichia coli exposed to Cd2+. J Bacteriol. 1978 Jan;133(1):75–80. doi: 10.1128/jb.133.1.75-80.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R. S., Gray R. H., Chin B., Bernstein I. A. Molecular mechanisms of accommodation in Escherichia coli to toxic levels of Cd2+. J Bacteriol. 1975 Mar;121(3):1180–1188. doi: 10.1128/jb.121.3.1180-1188.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R. S. Protein synthesis in Escherichia coli during recovery from exposure to low levels of Cd2+. Appl Environ Microbiol. 1984 May;47(5):1012–1016. doi: 10.1128/aem.47.5.1012-1016.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafson R. W., Loya S., Sim R. G. Physiological parameters of prokaryotic metallothionein induction. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1495–1503. doi: 10.1016/s0006-291x(80)80066-0. [DOI] [PubMed] [Google Scholar]
- Pickett A. W., Dean A. C. Cadmium and zinc sensitivity and tolerance in Klebsiella (Aerobacter) aerogenes. Microbios. 1976;15(60):79–91. [PubMed] [Google Scholar]
- Silver S., Clark D. Magnesium transport in Escherichia coli. J Biol Chem. 1971 Feb 10;246(3):569–576. [PubMed] [Google Scholar]
- Silver S., Johnseine P., King K. Manganese Active Transport in Escherichia coli. J Bacteriol. 1970 Dec;104(3):1299–1306. doi: 10.1128/jb.104.3.1299-1306.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Gos Z., Zajac J. Energy-dependent efflux of cadmium coded by a plasmid resistance determinant in Staphylococcus aureus. J Bacteriol. 1981 Aug;147(2):313–319. doi: 10.1128/jb.147.2.313-319.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Gos Z., Zajac J. Reduced cadmium transport determined by a resistance plasmid in Staphylococcus aureus. J Bacteriol. 1981 Aug;147(2):305–312. doi: 10.1128/jb.147.2.305-312.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Ulmer D. D. Biochemical effects of mercury, cadmium, and lead. Annu Rev Biochem. 1972;41(10):91–128. doi: 10.1146/annurev.bi.41.070172.000515. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Silver S., Kinscherf T. G. Cation transport alteration associated with plasmid-determined resistance to cadmium in Staphylococcus aureus. Antimicrob Agents Chemother. 1978 Dec;14(6):856–865. doi: 10.1128/aac.14.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]



