Abstract
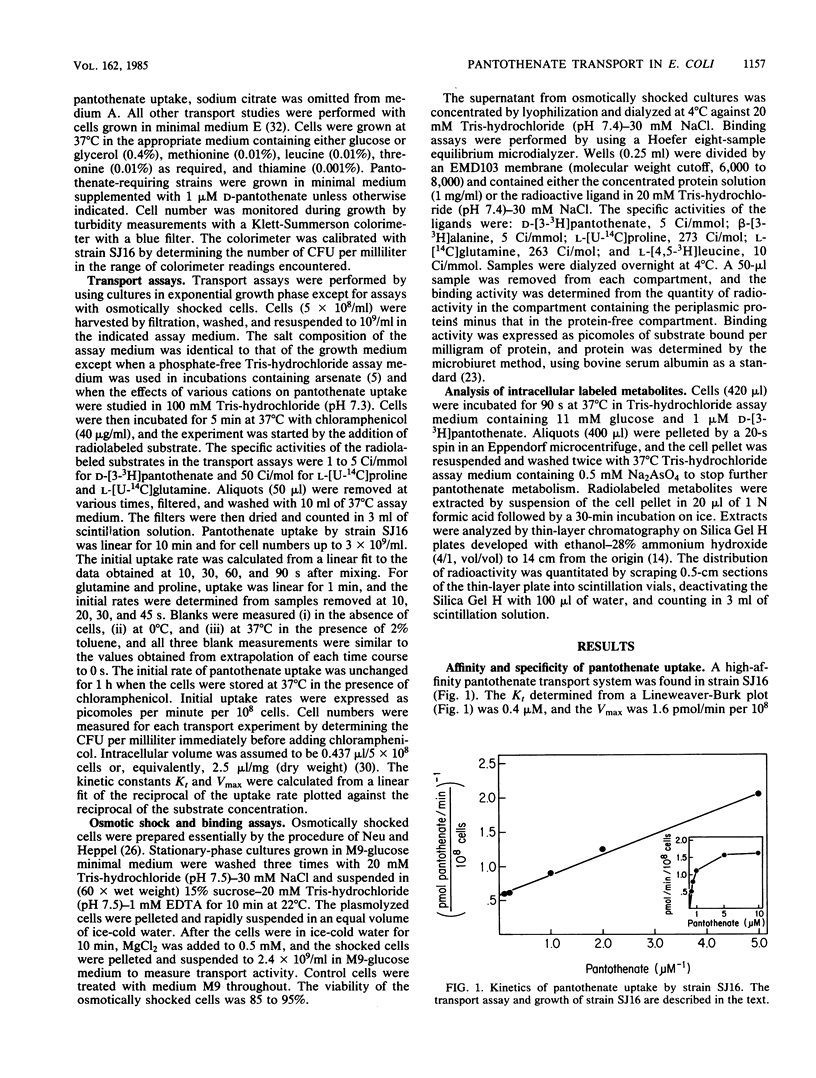
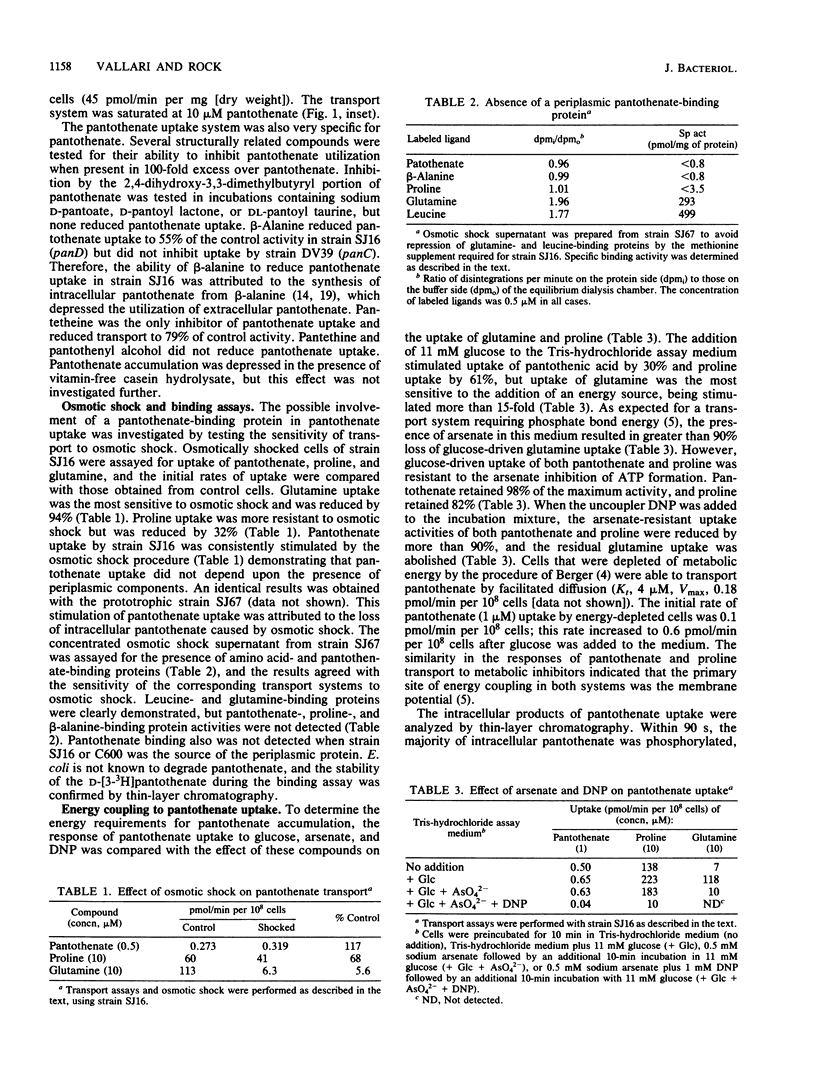
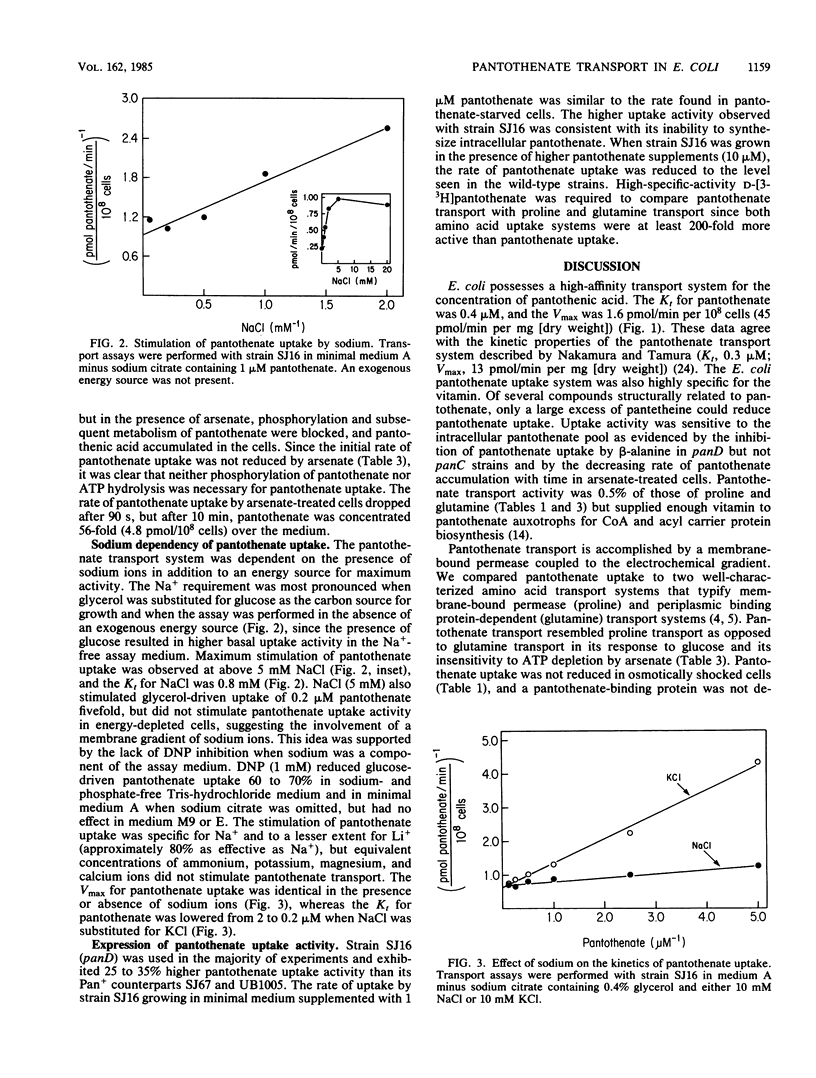
The transport system for pantothenic acid uptake in Escherichia coli was characterized. This transport system was specific for pantothenate, had a Kt of 0.4 microM, and had a maximum velocity of 1.6 pmol/min per 10(8) cells (45 pmol/min per mg [dry weight]). Pantothenate uptake was not reduced in osmotically shocked cells or by ATP depletion with arsenate, but was reduced greater than 90% by the dissipation of the membrane electrochemical gradient with 2,4-dinitrophenol. Sodium ions stimulated pantothenate uptake (Kt, 0.8 mM) by reducing the Kt for pantothenate by an order of magnitude. Intracellular pantothenate was rapidly phosphorylated, but phosphorylation of pantothenate was not required for uptake since pantothenate was the only labeled intracellular compound concentrated by ATP-depleted, glucose-energized cells. The data were consistent with the presence of a high-affinity pantothenate permease that concentrates the vitamin by sodium cotransport.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abiko Y., Ashida S. I., Shimizu M. Purification and properties of D-pantothenate kinase from rat liver. Biochim Biophys Acta. 1972 May 12;268(2):364–372. doi: 10.1016/0005-2744(72)90331-2. [DOI] [PubMed] [Google Scholar]
- BROWN G. M. The metabolism of pantothenic acid. J Biol Chem. 1959 Feb;234(2):370–378. [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Cronan J. E., Jr, Littel K. J., Jackowski S. Genetic and biochemical analyses of pantothenate biosynthesis in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1982 Mar;149(3):916–922. doi: 10.1128/jb.149.3.916-922.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom C. B., Canellas P. F., Wedding R. T. Allosteric regulation of the NAD malic enzyme from cauliflower: activation by fumarate and coenzyme A. Arch Biochem Biophys. 1983 Jan;220(1):133–144. doi: 10.1016/0003-9861(83)90394-6. [DOI] [PubMed] [Google Scholar]
- Halpern Y. S., Barash H., Dover S., Druck K. Sodium and potassium requirements for active transport of glutamate by Escherichia coli K-12. J Bacteriol. 1973 Apr;114(1):53–58. doi: 10.1128/jb.114.1.53-58.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorsen O., Skrede S. Regulation of the biosynthesis of CoA at the level of pantothenate kinase. Eur J Biochem. 1982 May;124(1):211–215. doi: 10.1111/j.1432-1033.1982.tb05927.x. [DOI] [PubMed] [Google Scholar]
- Hampsey D. M., Kohlhaw G. B. Inactivation of yeast alpha-isopropylmalate synthase by CoA. Antagonism between CoA and adenylates and the mechanism of CoA inactivation. J Biol Chem. 1981 Apr 25;256(8):3791–3796. [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Metabolism of 4'-phosphopantetheine in Escherichia coli. J Bacteriol. 1984 Apr;158(1):115–120. doi: 10.1128/jb.158.1.115-120.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Regulation of coenzyme A biosynthesis. J Bacteriol. 1981 Dec;148(3):926–932. doi: 10.1128/jb.148.3.926-932.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa T., Yoshida K., Furukawa K., Hosoki K. Feedback inhibition of pantothenate kinase by coenzyme A and possible role of the enzyme for the regulation of cellular coenzyme A level. J Biochem. 1972 Jun;71(6):1065–1067. doi: 10.1093/oxfordjournals.jbchem.a129854. [DOI] [PubMed] [Google Scholar]
- Lent B. A., Kim K. H. Requirement of acetyl-coenzyme A carboxylase kinase for coenzyme A. Arch Biochem Biophys. 1983 Sep;225(2):964–971. doi: 10.1016/0003-9861(83)90112-1. [DOI] [PubMed] [Google Scholar]
- MAAS W. K. Pantothenate studies. III. Description of the extracted pantothenate-synthesizing enzyme of Escherichia coli. J Biol Chem. 1952 Sep;198(1):23–32. [PubMed] [Google Scholar]
- MUNKRES K. D., RICHARDS F. M. THE PURIFICATION AND PROPERTIES OF NEUROSPORA MALATE DEHYDROGENASE. Arch Biochem Biophys. 1965 Mar;109:466–479. doi: 10.1016/0003-9861(65)90391-7. [DOI] [PubMed] [Google Scholar]
- McIlwain H. The metabolism and functioning of vitamin-like compounds: 2. A comparison of pantothenate metabolism by proliferating and by non-proliferating bacteria. Biochem J. 1946;40(2):269–278. [PMC free article] [PubMed] [Google Scholar]
- Mäntsälä P. Some characteristics and control of pantothenate transport in Escherichia coli U-5-41. Acta Chem Scand. 1973;27(2):445–452. doi: 10.3891/acta.chem.scand.27-0445. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Tamura Z. Pantothenate uptake in Escherichia coli K-12. J Nutr Sci Vitaminol (Tokyo) 1973 Oct;19(5):389–400. doi: 10.3177/jnsv.19.389. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Horecker B. L., Traniello S., Pontremoli S. Rabbit liver and rabbit kidney fructose diphosphatases: catalytic properties of enzymes activated by coenzyme A and acyl carrier protein. Arch Biochem Biophys. 1970 Jul;139(1):190–199. doi: 10.1016/0003-9861(70)90060-3. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Penrose W. R., Nichoalds G. E., Piperno J. R., Oxender D. L. Purification and properties of a leucine-binding protein from Escherichia coli. J Biol Chem. 1968 Nov 25;243(22):5921–5928. [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino-acid-binding protein released from Escherichia coli by osmotic shock. J Biol Chem. 1966 Dec 10;241(23):5732–5734. [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Stock J., Roseman S. A sodium-dependent sugar co-transport system in bacteria. Biochem Biophys Res Commun. 1971 Jul 2;44(1):132–138. doi: 10.1016/s0006-291x(71)80168-7. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wilson D. B. Cellular transport mechanisms. Annu Rev Biochem. 1978;47:933–965. doi: 10.1146/annurev.bi.47.070178.004441. [DOI] [PubMed] [Google Scholar]
- Yeh L. A., Kim K. H. Regulation of acetyl-coA carboxylase: properties of coA activation of acetyl-coA carboxylase. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3351–3355. doi: 10.1073/pnas.77.6.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh L. A., Song C. S., Kim K. H. Coenzyme A activation of acetyl-CoA carboxylase. J Biol Chem. 1981 Mar 10;256(5):2289–2296. [PubMed] [Google Scholar]



