Abstract
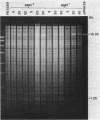
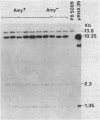
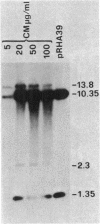
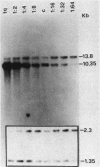
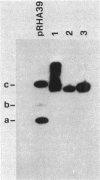
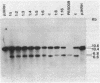
We report on the amplification in Bacillus subtilis of a defined DNA sequence after exposure of the bacteria to increasing levels of antibiotic. The experimental system consisted of transformation of competent cells with a plasmid (pRHA39) unable to replicate in the host and carrying the alpha-amylase gene derived from B. subtilis. Selection of transformants resistant to 5 micrograms of chloramphenicol per ml resulted in the isolation of strains with the plasmid integrated into the chromosome at the site of homology, by a Campbell type mechanism. Starting from such a nontandem duplication, amplification was achieved by growing the bacteria in increasing concentrations of chloramphenicol. By dilution, Southern blotting, and hybridization to a radioactive probe, we estimated a copy number of about 10 for the amplified sequence of samples grown in the presence of 50 micrograms of chloramphenicol per ml. No free plasmid could be detected in the amplified strains. The extent of the amplified region was the same for all transformants, and the endpoints appeared to be the same in all isolates. As a consequence of the amplification, there was a noticeable increase in amylase production, and the amount of enzyme produced correlated with gene dosage. The amplification did not occur in a recE genetic background.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Roth J. Spontaneous tandem genetic duplications in Salmonella typhimurium arise by unequal recombination between rRNA (rrn) cistrons. Proc Natl Acad Sci U S A. 1981 May;78(5):3113–3117. doi: 10.1073/pnas.78.5.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. P., Roth J. R. Tandem chromosomal duplications in Salmonella typhimurium: fusion of histidine genes to novel promoters. J Mol Biol. 1978 Feb 15;119(1):147–166. doi: 10.1016/0022-2836(78)90274-7. [DOI] [PubMed] [Google Scholar]
- Anderson R. P., Roth J. R. Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol. 1977;31:473–505. doi: 10.1146/annurev.mi.31.100177.002353. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chak K. F., de Lencastre H., Liu H. M., Piggot P. J. Facile in vivo transfer of mutations between the Bacillus subtilis chromosome and a plasmid harbouring homologous DNA. J Gen Microbiol. 1982 Nov;128(11):2813–2816. doi: 10.1099/00221287-128-11-2813. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Duncan C. H., Wilson G. A., Young F. E. Mechanism of integrating foreign DNA during transformation of Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3664–3668. doi: 10.1073/pnas.75.8.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund T., Normark S. Recombination between short DNA homologies causes tandem duplication. Nature. 1981 Jul 16;292(5820):269–271. doi: 10.1038/292269a0. [DOI] [PubMed] [Google Scholar]
- Ehrlich S. D. DNA cloning in Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1433–1436. doi: 10.1073/pnas.75.3.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari F. A., Nguyen A., Lang D., Hoch J. A. Construction and properties of an integrable plasmid for Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1513–1515. doi: 10.1128/jb.154.3.1513-1515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizzi A., Scoffone F., Milanesi G., Albertini A. M. Integration and excision of a plasmid in Bacillus subtilis. Mol Gen Genet. 1981;182(1):99–105. doi: 10.1007/BF00422774. [DOI] [PubMed] [Google Scholar]
- Gutterson N. I., Koshland D. E., Jr Replacement and amplification of bacterial genes with sequences altered in vitro. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4894–4898. doi: 10.1073/pnas.80.16.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldenwang W. G., Banner C. D., Ollington J. F., Losick R., Hoch J. A., O'Connor M. B., Sonenshein A. L. Mapping a cloned gene under sporulation control by inserttion of a drug resistance marker into the Bacillus subtilis chromosome. J Bacteriol. 1980 Apr;142(1):90–98. doi: 10.1128/jb.142.1.90-98.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick R. M., Lederberg J. Interspecies transformation in Bacillus: mechanism of heterologous intergenote transformation. J Bacteriol. 1978 Mar;133(3):1246–1253. doi: 10.1128/jb.133.3.1246-1253.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S., Yamane K., Sasaki T., Yamasaki M., Tamura G., Maruo B. Tunicamycin-resistant mutants and chromosomal locations of mutational sites in Bacillus subtilis. J Bacteriol. 1978 Nov;136(2):818–821. doi: 10.1128/jb.136.2.818-821.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport G., Klier A., Billault A., Fargette F., Dedonder R. Construction of a colony bank of E. coli containing hybrid plasmids representative of the Bacillus subtilis 168 genome. Expression of functions harbored by the recombinant plasmids in B. subtilis. Mol Gen Genet. 1979 Oct 3;176(2):239–245. doi: 10.1007/BF00273218. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Stahl M. L., Ferrari E. Replacement of the Bacillus subtilis subtilisin structural gene with an In vitro-derived deletion mutation. J Bacteriol. 1984 May;158(2):411–418. doi: 10.1128/jb.158.2.411-418.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G. R., Wahl G. M. Gene amplification. Annu Rev Biochem. 1984;53:447–491. doi: 10.1146/annurev.bi.53.070184.002311. [DOI] [PubMed] [Google Scholar]
- Tlsty T. D., Albertini A. M., Miller J. H. Gene amplification in the lac region of E. coli. Cell. 1984 May;37(1):217–224. doi: 10.1016/0092-8674(84)90317-9. [DOI] [PubMed] [Google Scholar]
- Yang M., Galizzi A., Henner D. Nucleotide sequence of the amylase gene from Bacillus subtilis. Nucleic Acids Res. 1983 Jan 25;11(2):237–249. doi: 10.1093/nar/11.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. Gene amplification in Bacillus subtilis. J Gen Microbiol. 1984 Jul;130(7):1613–1621. doi: 10.1099/00221287-130-7-1613. [DOI] [PubMed] [Google Scholar]
- Young M. The mechanism of insertion of a segment of heterologous DNA into the chromosome of Bacillus subtilis. J Gen Microbiol. 1983 May;129(5):1497–1512. doi: 10.1099/00221287-129-5-1497. [DOI] [PubMed] [Google Scholar]