Abstract
Plasmids of incompatibility group HI are large (greater than 150 kilobases [kb]) and possess an unusual thermosensitive mode of conjugative transfer. R27, the prototype IncHi1 plasmid, encodes resistance to tetracycline via a determinant which is related to transposon Tn10. A restriction endonuclease map of R27 (size, 182 kb) was recently constructed with ApaI, PstI, and XbaI. Transfer genes within R27 were mapped by insertion of Tn5 and Tn7. At least two different regions of the plasmid were concerned with transfer functions. Insertions into either region completely abolished transfer. None of the insertions had any effect on entry exclusion (Eex) of other IncH plasmids. However, a deletion mutant which lacked the Eex function was obtained, allowing us to map the probable site of the gene encoding Eex to one of the two transfer regions. The tetracycline resistance determinant in R27 was located within an 8-kb region between the two main transfer regions. The transfer genes, therefore, are not located together in R27 but are situated in at least two major widely separated transfer regions.
Full text
PDF
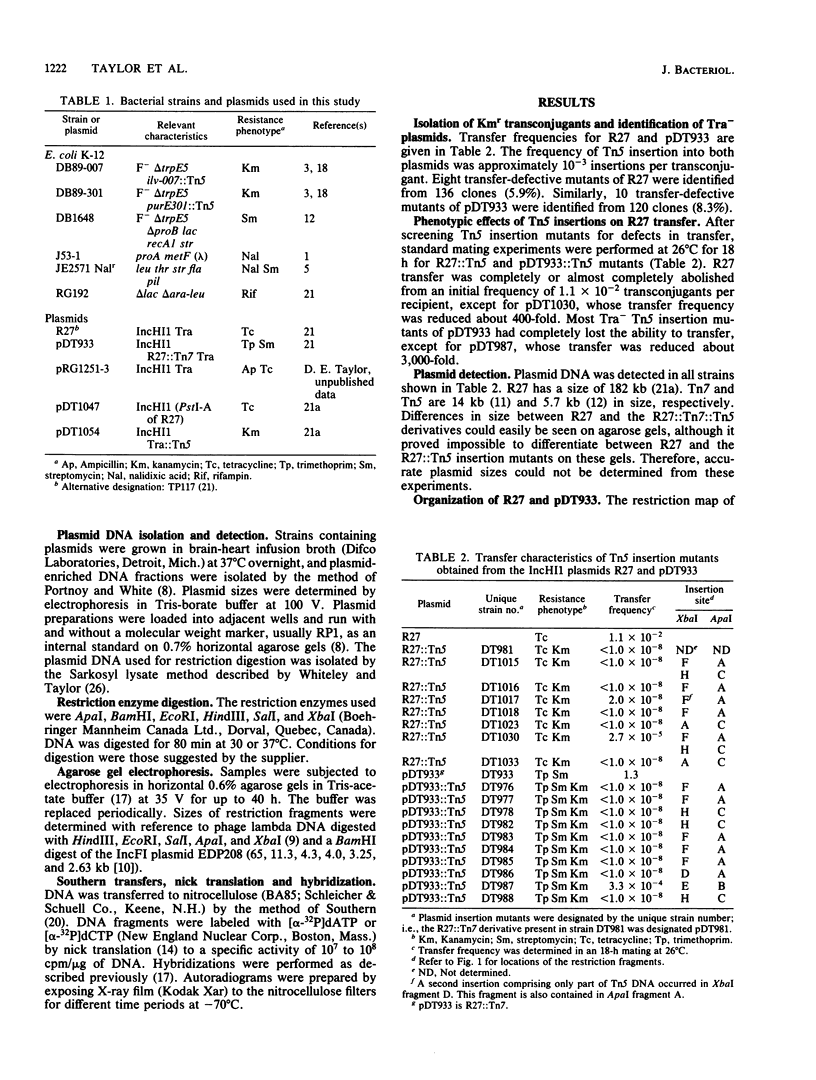
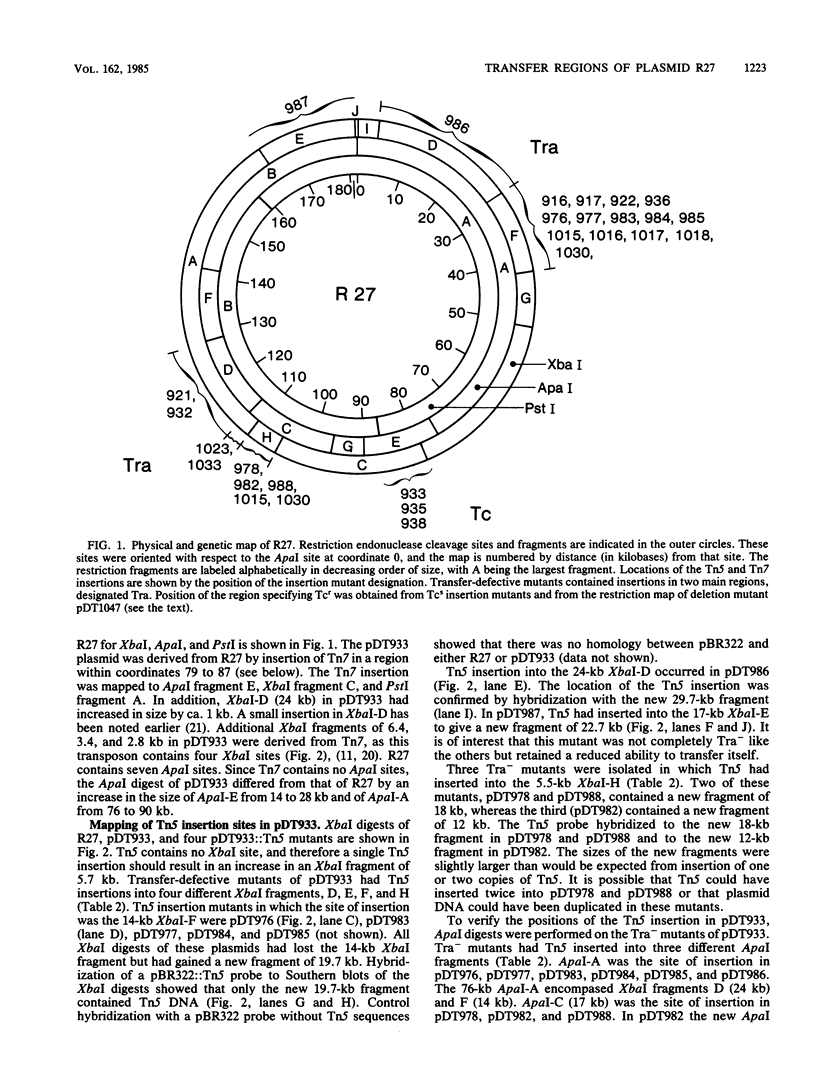
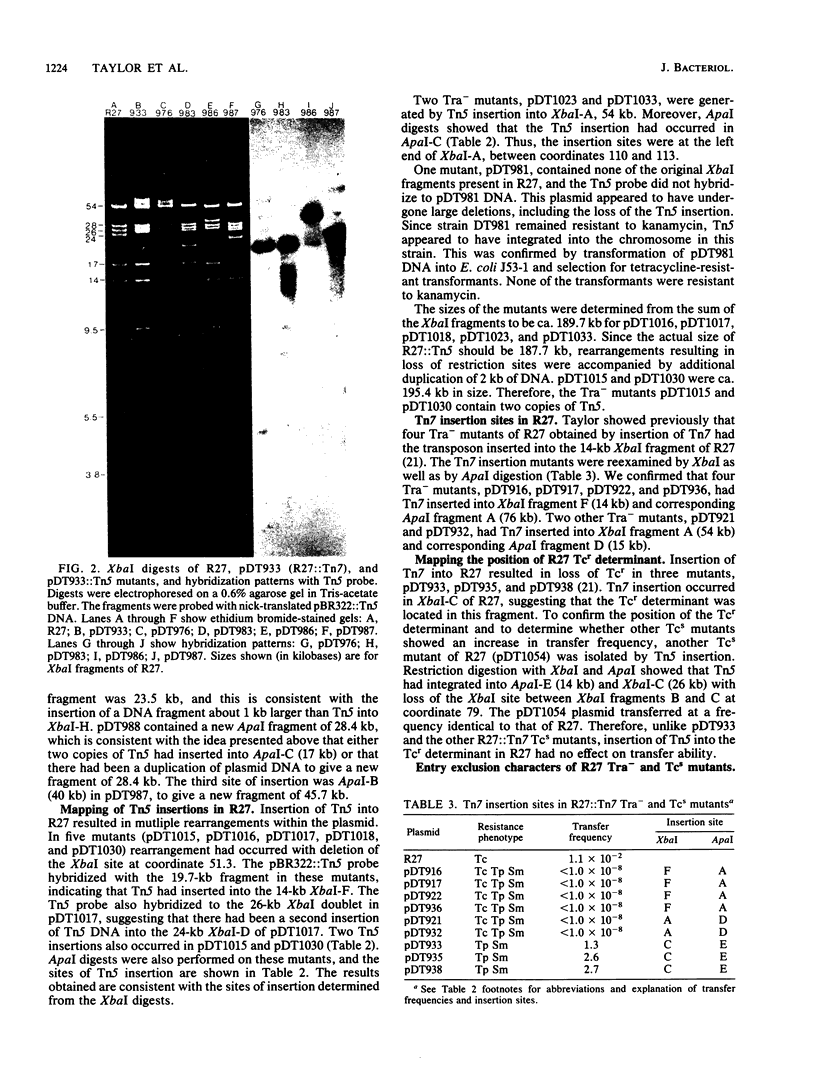


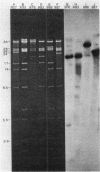
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Weiss A., Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980 May;142(2):439–446. doi: 10.1128/jb.142.2.439-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Determination of pili by conjugative bacterial drug resistance plasmids of incompatibility groups B, C, H, J, K, M, V, and X. J Bacteriol. 1980 Feb;141(2):828–837. doi: 10.1128/jb.141.2.828-837.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E., Taylor D. E., Cohen D. R. Specification of surface mating systems among conjugative drug resistance plasmids in Escherichia coli K-12. J Bacteriol. 1980 Sep;143(3):1466–1470. doi: 10.1128/jb.143.3.1466-1470.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Willetts N. S. A physical and genetic map of the IncN plasmid R46. Plasmid. 1981 Mar;5(2):188–201. doi: 10.1016/0147-619x(81)90020-2. [DOI] [PubMed] [Google Scholar]
- Chilton M. D., Montoya A. L., Merlo D. J., Drummond M. H., Nutter R., Gordon M. P., Nester E. W. Restriction endonuclease mapping of a plasmid that confers oncogenicity upon Agrobacterium tumefaciens strain B6-806. Plasmid. 1978 Feb;1(2):254–269. doi: 10.1016/0147-619x(78)90043-4. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Paranchych W., Falkow S. Characterization of conjugative plasmid EDP208. J Bacteriol. 1983 Oct;156(1):230–235. doi: 10.1128/jb.156.1.230-235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosti-Testu F., Norris V., Brevet J. Restriction map of Tn7. Plasmid. 1983 Jul;10(1):96–99. doi: 10.1016/0147-619x(83)90061-6. [DOI] [PubMed] [Google Scholar]
- Hirschel B. J., Berg D. E. A derivative of Tn5 with direct terminal repeats can transpose. J Mol Biol. 1982 Feb 25;155(2):105–120. doi: 10.1016/0022-2836(82)90439-9. [DOI] [PubMed] [Google Scholar]
- Holsters M., Silva B., Van Vliet F., Genetello C., De Block M., Dhaese P., Depicker A., Inzé D., Engler G., Villarroel R. The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid. 1980 Mar;3(2):212–230. doi: 10.1016/0147-619x(80)90110-9. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez B., Tachibana C., Levy S. B. Heterogeneity of tetracycline resistance determinants. Plasmid. 1980 Mar;3(2):99–108. doi: 10.1016/0147-619x(80)90101-8. [DOI] [PubMed] [Google Scholar]
- Moore R. J., Krishnapillai V. Tn7 and Tn501 Insertions into Pseudomonas aeruginosa plasmid R91-5: mapping of two transfer regions. J Bacteriol. 1982 Jan;149(1):276–283. doi: 10.1128/jb.149.1.276-283.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K. J., Berg C. M. Escherichia coli K-12 auxotrophs induced by insertion of the transposable element Tn5. Genetics. 1979 Jul;92(3):741–747. doi: 10.1093/genetics/92.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W. Letter: Thermosensitive transfer factors in chloramphenicol-resistant strains of Salmonella typhi. Lancet. 1974 Aug 3;2(7875):281–282. doi: 10.1016/s0140-6736(74)91435-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Brose E. C. Restriction endonuclease mapping of R27 (TP117), an incompatibility group HI subgroup 1 plasmid from Salmonella typhimurium. Plasmid. 1985 Jan;13(1):75–77. doi: 10.1016/0147-619x(85)90058-7. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Grant R. B. Incompatibility and surface exclusion properties of H1 and H2 plasmids. J Bacteriol. 1977 Jul;131(1):174–178. doi: 10.1128/jb.131.1.174-178.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Levine J. G. Studies of temperature-sensitive transfer and maintenance of H incompatibility group plasmids. J Gen Microbiol. 1980 Feb;116(2):475–484. doi: 10.1099/00221287-116-2-475. [DOI] [PubMed] [Google Scholar]
- Taylor D. E. Transfer-defective and tetracycline-sensitive mutants of the incompatibility group HI plasmid R27 generated by insertion of transposon 7. Plasmid. 1983 May;9(3):227–239. doi: 10.1016/0147-619x(83)90001-x. [DOI] [PubMed] [Google Scholar]
- Tempé J., Petit A., Holsters M., Montagu M., Schell J. Thermosensitive step associated with transfer of the Ti plasmid during conjugation: Possible relation to transformation in crown gall. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2848–2849. doi: 10.1073/pnas.74.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatte V., Iyer V. N. Cloning of a plasmid region specifying the N transfer system of bacterial conjugation in Escherichia coli. Gene. 1983 Mar;21(3):227–236. doi: 10.1016/0378-1119(83)90006-9. [DOI] [PubMed] [Google Scholar]
- Whiteley M., Taylor D. E. Identification of DNA homologies among H incompatibility group plasmids by restriction enzyme digestion and Southern transfer hybridization. Antimicrob Agents Chemother. 1983 Aug;24(2):194–200. doi: 10.1128/aac.24.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Skurray R. The conjugation system of F-like plasmids. Annu Rev Genet. 1980;14:41–76. doi: 10.1146/annurev.ge.14.120180.000353. [DOI] [PubMed] [Google Scholar]