Abstract
RNA-mediated virus resistance has recently been shown to be the result of post-transcriptional transgene silencing in transgenic plants. This study was undertaken to characterize the effect of transgene length and nontarget DNA sequences on RNA-mediated tospovirus resistance in transgenic plants. Transgenic Nicotiana benthamiana plants were generated to express different regions of the nucleocapsid (N) protein of tomato spotted wilt (TSWV) tospovirus. Transgenic plants expressing half-gene segments (387–453 bp) of the N gene displayed resistance through post-transcriptional gene silencing. Although smaller N gene segments (92–235 bp) were ineffective in conferring resistance when expressed alone in transgenic plants, these segments conferred resistance when fused to the nontarget green fluorescent protein gene DNA. These results demonstrate that (i) a critical length of N transgene (236–387 bp) is required for a high level of transgene expression and consequent gene silencing, and (ii) the post-transcriptional gene silencing mechanism can trans-inactivate the incoming tospovirus genome with homologous transgene segments that are as short as 110 bp. Therefore, the activation of post-transcriptional transgene silencing requires a significantly larger transgene than is required for the trans-inactivation of the incoming viral genome. These results raise the possibility of developing a simple new strategy for engineering multiple virus resistance in transgenic plants.
Keywords: pathogen-derived resistance, cosuppression
Gene silencing was discovered when introduction of a transgene resulted in suppression of the homologous host gene (1, 2). Subsequent studies showed that transgenes can also be silenced when multiple copies of the transgene were introduced into plants or when a single copy of the transgene became homozygous (3–10). Gene silencing can occur at post-transcriptional or transcriptional levels (3, 11–14). Post-transcriptional gene silencing results from an increase in RNA turnover (3, 15). Transcriptional gene silencing usually involves extensive methylation of the affected genes (4, 7, 9, 12, 16–18). However, transgene methylation is not limited to transcriptional gene silencing and has recently been reported to be also associated with post-transcriptional gene silencing (14, 19–22).
Post-transcriptional silencing has recently been found to be the cause of RNA-mediated virus resistance in transgenic plants (20–28). This type of virus resistance is also referred to as homology-dependent resistance (25, 29). RNA-mediated resistance is effective against RNA viruses that replicate and accumulate in the cytoplasm of the infected cells. It is therefore likely that the mechanism of gene silencing and RNA-mediated virus resistance takes place in the cytoplasm. Several models have been postulated to explain observed gene silencing and the resulting virus resistance, which include the expression threshold model (8, 20, 24, 30, 31) and the ectopic pairing model (22, 31, 32).
Previously, we generated engineered resistance to tomato spotted wilt virus (TSWV) in lettuce (27), tobacco (33, 34), Nicotiana benthamiana (35), and tomato (unpublished work). The genome of tospoviruses consists of three single-stranded RNAs: S RNA (2,900 nucleotides), M RNA (≈5,000 nucleotides), and L RNA (8,900 nucleotides). Both S and M RNAs contain two open reading frames (ORFs) of an ambisense gene arrangement (36–41), which are expressed by means of the synthesis of subgenomic mRNAs (42). The S RNA encodes a 52-kDa nonstructural protein (NSs) in the viral RNA strand and the 29-kDa nucleocapsid (N) protein in the viral complementary RNA strand, while the M RNA encodes a 34-kDa nonstructural protein (NSm) in the viral RNA strand and the precursor to the 58-kDa (G2) and 78-kDa (G1) membrane-associated glycoproteins in the viral complementary RNA strand. The L RNA is of negative polarity and encodes a large 200-kDa protein, presumably for the viral transcriptase (43).
We are studying post-transcriptional gene silencing in relation to the consequent tospovirus resistance in transgenic plants. Previously, we reported that this type of resistance is affected by transgene dosage and the stage of plant development in transgenic lettuce expressing full-length N gene sequences (27). Here we demonstrate that the RNA-mediated tospovirus resistance is dependent on the length of the N transgene. Unlike large N gene segments (387–453 bp), small N transgene segments (92–235 bp) were not silenced in transgenic plants unless they were transcriptionally fused to the green fluorescent protein gene (GFP). The implication of this observation is discussed in relation to the possible mechanism of post-transcriptional gene silencing as well as a possible strategy for engineering multiple virus resistance in transgenic plants.
MATERIALS AND METHODS
Cloning and Transformation.
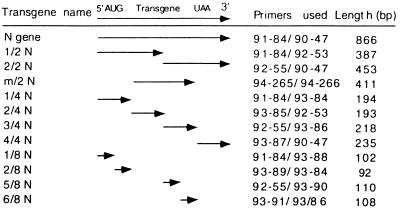
The N gene (33) of the lettuce isolate of tomato spotted wilt virus (TSWV-BL) was used as the template for construction of the N gene segments of various lengths using the primers listed in Table 1. The forward primers for the N gene segments were designed to contain an out-of-frame ATG and/or stop codons to ensure the production of untranslatable N gene transcripts. The PCR-amplified N gene segments (Table 2) were cloned in the sense orientation into the NcoI site of the plant expression vector pBI525 (33).
Table 1.
Primers used in cloning N gene segments of TSWV and the entire GFP gene
| Name | Nucleotide positions* | Sequence |
|---|---|---|
| For the N gene fragments | ||
| 91-84 | 2766–2744 | 5′-AGCTAATCTAGAACCATGGATGACTCACTAAGGAAAGCATTGTTGC |
| 93-89 | 2669–2650 | 5′-TACAGTTCTAGAACCATGGTCTGGAAAACCTTGACCAG |
| 93-85 | 2576–2556 | 5′-TACAGTTCTAGAACCATGGTAAAGCGATTTTACTTTTGGTA |
| 92-55 | 2373–2354 | 5′-AGATTCTCTAGACCATGGTGACTTGATGAGCAAAGTCTGTGAGGCTTGC |
| 93-91 | 2266–2248 | 5′-TACAGTTCTAGAACCATGGAAAATACAAGGATCTCGGG |
| 93-87 | 2153–2133 | 5′-TACAGTTCTAGAACCATGGTAGAAGGGGAAAGAGTATGCTG |
| 90-47 | 1918–1937 | 5′-AGCATTGGATCCATGGTTAACACACTAAGCAAGCAC |
| 93-86 | 2158–2177 | 5′-TCTTGAGGATCCATGGCTGATCTTCATTCATTTCAA |
| 93-90 | 2269–2288 | 5′-TCTTGAGGATCCATGGATCCTGATATATAGCCAAGA |
| 92-53 | 2383–2402 | 5′-TACAGTGGATCCATGGTTAAGGTAATCCATAGGCTTGAC |
| 93-84 | 2577–2597 | 5′-TCTTGAGGATCCATGGCTTAATAACCTTCATTATGC |
| 93-88 | 2671–2690 | 5′-TCTTGAGGATCCATGGAAAAGTCTTGAAGTTGAATG |
| 94-265 | 2556–2530 | 5′-AGCTAATCTAGAACCATGGATGAAAAATTACCATAAAGAAAACTTCAGAC |
| 94-266 | 2182–2206 | 5′-AGCATTGGATCCATGGTTAGTTACCTAGTTTTCTTTTCAGCACAGTGCAAACT |
| For the GFP gene | ||
| 5′ GFP | NA | 5′-TGAACATCTAGAACCATGGGTAAAGGAGAAGAACTTTTCACTGG |
| 3′ GFP | NA | 5′-TGAACAGGATCCATGGTCTACGAATGCTATTATTTGTATAGTTC |
NA, not applicable.
Nucleotide position number of each primer for the N gene segments was as published by Pang et al. (33). The N gene and GFP gene sequences are underlined and the rest of the sequences correspond to the primers used for cloning. Out-of-frame ATG and stop codons for the untranslatable versions in the 5′ primers are shown in boldface.
Table 2.
Inoculations of TSWV-BL to R1 N. benthamiana plants expressing N gene segments
| Gene | Size, bp | No. tested
|
No. of resistant lines | Reactions of test plants*
|
|||
|---|---|---|---|---|---|---|---|
| Lines | Plants | HS | HT | HR | |||
| 1/2 N | 387 | 5 | 99 | 2 | 75 | 4 | 20 |
| m/2 N | 411 | 6 | 108 | 6 | 36 | 17 | 55 |
| 2/2 N | 453 | 7 | 118 | 5 | 63 | 26 | 29 |
| 1/4 N | 194 | 12 | 134 | 0 | 134 | ||
| 2/4 N | 193 | 5 | 64 | 0 | 64 | ||
| 3/4 N | 218 | 12 | 145 | 0 | 145 | ||
| 4/4 N | 235 | 7 | 89 | 0 | 89 | ||
| 1/8 N | 102 | 14 | 170 | 0 | 170 | ||
| 2/8 N | 92 | 8 | 63 | 0 | 63 | ||
| 5/8 N | 110 | 7 | 85 | 0 | 85 | ||
| 6/8 N | 108 | 13 | 162 | 0 | 162 | ||
| Control | 239 | 239 | |||||
TSWV-BL-infected leaf extracts (1/30) were applied to three upper leaves of N. benthamiana at the 5- to 7-leaf stage. Reactions were grouped into three phenotypes: (i) highly susceptible (HS), typical systemic symptoms were observed 5–10 days after inoculation; (ii) highly tolerant (HT), systemic symptoms were delayed more than 10 days after inoculation; and (iii) highly resistant (HR), plants remained symptomless. All R1 plants were tested by nptII ELISA to identify transgenic plants. Nontransgenic plants are in the control row; the other rows consist of transgenic plants. See Fig. 1 for definitions of N gene segments of TSWV-BL.
For construction of various N gene segment fusions with GFP, the translatable GFP ORF was amplified with GFP primers (Table 1) from the plasmid pGFP (CLONTECH) and cloned as transcriptional fusions into the 5′ NcoI site of the N gene segments 2/2 N, 3/4 N, and 5/8 N in pBI525. The resulting GFP/N fusions contained translatable GFP ORF followed by untranslatable N gene segments of different lengths as the 3′ untranslated regions of the GFP gene.
The resulting plant expression vectors were digested with HindIII and EcoRI (partial digestion where necessary), and the HindIII–EcoRI segments containing the corresponding gene cassettes were isolated and introduced into the same sites of pBIN19. The resulting binary vectors were transferred into Agrobacterium tumefaciens LBA4404, and the A. tumefaciens containing the vectors were used to inoculate leaf discs of Nicotiana benthamiana plants, essentially as described by Horsch et al. (44).
ELISA and Northern Blot Analyses of Transgenic Plants.
Double-antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) was used to detect the nptII enzyme in transgenic plants using an nptII ELISA kit (5 Prime → 3 Prime). Northern blotting was performed as described previously (34).
Inoculation of Transgenic Plants.
Inoculations were done as described previously (33). Systemic symptoms were recorded every other day for at least 2 months.
Isolation of Nuclei and Nuclear Run-off Transcription Assays.
Isolation of nuclei and nuclear run-off transcription assays were previously described (27).
RESULTS
Small N Gene Segments (92–235 bp) Do Not Induce RNA-Mediated Tospovirus Resistance.
The untranslatable N gene segments 92–453 bp in length (Table 2) were PCR amplified using appropriate sets of the 5′ and 3′ primers (Table 1). The 5′ primers were designed to contain an out-of-frame ATG followed by stop codons to prevent the translation of truncated N protein. The N gene segments, representing various regions of the entire N gene (Fig. 1; Table 2), were cloned in the sense orientation into the plant expression vector pBI525. This vector utilized the enhanced cauliflower mosaic virus (CaMV) 35S promoter, the 5′ untranslated region from alfalfa mosaic virus (AlMV), and the 3′ untranslated region of the nopaline synthase gene. The resulting expression cassettes were used to obtain transgenic N. benthamiana plants by Agrobacterium-mediated transformation for this study.
Figure 1.
Map of the N transgenes of TSWV-BL that were used in this study.
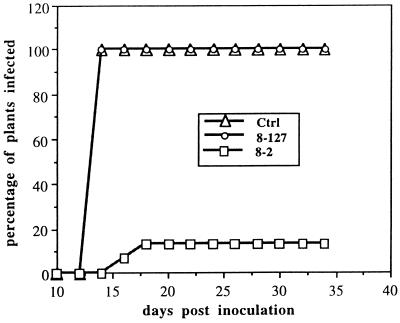
Transgenic R1 seedlings were initially screened with the nptII ELISA to identify nontransgenic segregants from the R1 transgenic populations. To exclude the possibility of escapes and avoid human bias during inoculation, nontransgenic segregants from each line were inoculated as internal controls along with transgenic R1 plants. Additionally, nontransformed N. benthamiana plants were also included in each inoculation experiment. Reactions of the inoculated R1 plants to TSWV-BL are summarized in Table 2. Unlike negative control plants, which were completely susceptible to the virus, expression of large N gene segments (387–453 bp, one-half N gene) conferred high levels of resistance to TSWV-BL in 20–51% of R1 plants and tolerance to tospovirus infection in 4–22% of R1 plants. The R1 plants were also shown to be resistant to a closely related TSWV-10W isolate but not to the distantly related impatiens necrotic spot virus (INSV) (39) or groundnut ringspot virus (GRSV) (41) isolates (data not shown). Northern analysis on selected lines showed correlation of the resistance phenotype with low levels of the N gene segment transcript accumulation (Figs. 2 and 3A). To confirm that the reduced steady-state mRNA levels of the N gene segments were due to post-transcriptional down-regulation of the transgene, nuclear run-off transcription analysis was performed. Using the endogenous actin as a control, the N gene segments were found to be transcribed in the silenced progenies at higher rates than in the nonsilenced high-expressing progenies (Fig. 3B). These results collectively suggested that the resistance that we observed was the result of post-transcriptional transgene silencing, which affected the steady-state mRNA accumulation but not transcription rate.
Figure 2.
Protection of transgenic plants against infection by TSWV-BL. R1 plants of selected lines (8-127, a 1/2 N gene high expressor and 8-2, a 1/2 N gene low expressor, as determined by Northern blotting) were assayed for nptII using an nptII ELISA kit. The ELISA negative nontransgenic segregates (0.00–0.02 OD405) were used as controls. The same plants were then challenged with TSWV-BL. Plants were examined every other day for the appearance of systemic symptoms, and any plant displaying symptoms on noninoculated leaves was recorded as systemic infection.
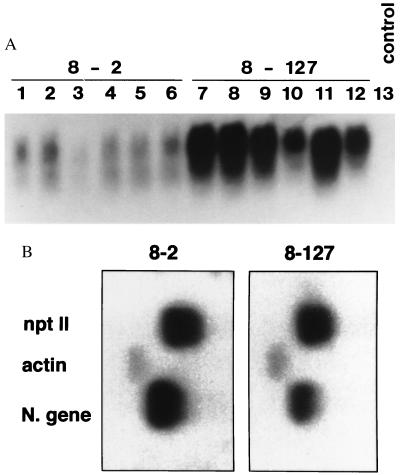
Figure 3.
Northern blot and nuclear run-off transcription analyses of 1/2 N gene low expressor (8-2) and high expressor (8-127) lines. (A) Northern blot analysis of 8-2 and 8-127 R1 plants. Total RNAs were isolated from transgenic plants (15 μg per lane) and analyzed by Northern blotting using as the probe the N gene of TSWV-BL. Lanes 1–6, R1 plants of line 8-2; lanes 7–12, R1 plants of line 8-127; lane 13, a nontransgenic control. Size of 1/2 N gene transcript is ≈670 bp. (B) Nuclear run-off transcription analysis on R1 plants. Labeled nuclear RNAs were hybridized to restriction enzyme-digested N gene, nptII, and actin fragments separated on agarose gels and blotted onto membranes. The nuclei used in the assays were isolated from an 8-2 R1 plant and an 8-127 R1 plant.
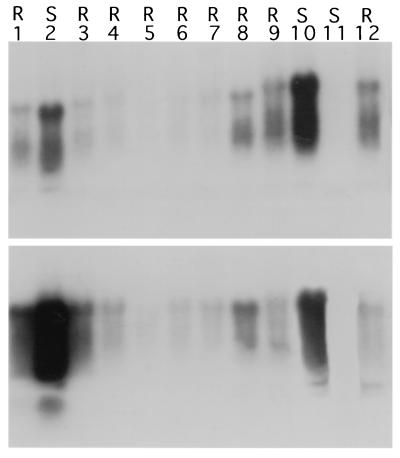
R1 plants expressing the small N gene segments (92–235 bp, one-fourth to one-eighth N gene) were similarly inoculated with TSWV-BL and other tospovirus isolates, and all of them were susceptible to tospovirus infection (Table 2). Northern blot analysis of selected R1 plants showed that the transcript level of the N gene segments correlated with the length of transgenes. As shown in Fig. 4, the transcripts of large transgenes generally accumulated at much higher levels than those of small transgenes in the nonsilenced R1 plants. This result suggested that the small N gene segments were not effectively transcribed in the nuclei, were transcribed at similar rates but were not properly processed or transported, or were not stable in the cytoplasm. As a result, there was no induction of transgene silencing in those small-transgene plants. In fact, a number of representative lines expressing the small N gene segments were analyzed by nuclear run-off transcription analysis, and the results showed no evidence that post-transcriptional silencing took place in any of those tested lines (data not shown).
Figure 4.
Comparison of transcript accumulations in plants expressing N gene segments of different lengths that had been tested for resistance and post-transcriptional gene silencing. Ten micrograms per lane of total RNAs isolated from transgenic plants was used in Northern blots which were probed with the N gene of TSWV-BL. Lanes 1 and 2, two nonsilenced, virus-susceptible R1 plants with the full-length N gene; lane 3, a silenced, virus-resistant R1 plant with 2/2 N gene; lane 4, a nonsilenced, virus-susceptible R1 plant with 2/2 N gene; lane 5, a nontransformed control; lanes 6–9, four nonsilenced, virus-susceptible R1 plants with 3/4 N gene; lanes 10–13, four nonsilenced, virus-susceptible R1 plants with 5/8 N gene. Sizes of transcripts for full-length N gene, 2/2 N gene, 3/4 N gene, and 5/8 N gene are ≈1150 bp, ≈730 bp, ≈500 bp, and ≈390 bp, respectively.
Fusions of the Small N Gene Segments with GFP Confer RNA-Mediated Tospovirus Resistance.
It was possible that the inability of the small N gene segments to confer RNA-mediated resistance was because the transgenes were too small to be expressed and accumulate at high levels (e.g., inefficient transcription, process, improper transport, or less stability) or because the small N gene segments fell below the minimal length of homology for trans-inactivation of the incoming virus genome. Various N gene segments (110, 218, and 453 bp) were fused to the 3′ end of the GFP gene, as described in Materials and Methods. Expression of such fusions in plant cells produces transcripts consisting of functional GFP ORF immediately followed by the respective N segments as the 3′ untranslated region. R0 plants expressing these fusions were inoculated with the homologous isolate TSWV-BL, and inoculation results are summarized in Table 3. As a control, transgenic R0 plants expressing GFP alone displayed typically systemic symptom at 5–10 days post inoculation. On the other hand, all GFP/N fusions conferred various levels of resistance to TSWV-BL (Table 3), including the small N segments (110 bp and 218 bp) which provided no protection against TSWV-BL when expressed alone in plants (Table 2). R1 progenies from selected R0 lines were similarly inoculated, and they showed similar levels of protection against TSWV-BL (Table 4). The R1 plants were also shown to be resistant to a closely related TSWV-10W isolate but not to the distantly related INSV or GRSV isolates (data not shown). These results suggest that the GFP gene triggered gene silencing which degraded GFP and the small targeted N gene segments, and the homologous sequences of the incoming virus, resulting in the resistant state of the plant. However, the smallest GFP-5/8 N fusion (110 bp of homology) was less effective against TSWV-BL, as reflected by the lower number of R0 plants protected and the quality of protection (Tables 3 and 4), indicating that the 110 bp of nucleotide sequence may approach the shortest homology required for trans-inactivation and consequent virus resistance. In addition, some of R1 plants expressing the fusions were analyzed by Northern blotting using both N gene and GFP gene as probes (Fig. 5), and the results showed that the observed resistance again correlated with low accumulation levels of the fusion gene transcripts, suggesting that the same resistance mechanism operates in plants expressing the N gene segments alone or the GFP/N fusions.
Table 3.
Inoculations of TSWV-BL to R0 N. benthamiana plants transformed with GFP gene fused to N gene segments
| Genes | GFP + N, bp | No. of lines | Reactions of test plants
|
||
|---|---|---|---|---|---|
| HS | HT | HR | |||
| GFP | 720 + 0 | 8 | 8 | ||
| GFP + 5/8 N | 720 + 110 | 13 | 11 | 1 | 1 |
| GFP + 3/4 N | 720 + 218 | 8 | 2 | 6 | |
| GFP + 2/2 N | 720 + 453 | 14 | 5 | 1 | 8 |
Table 4.
TSWV-BL inoculations of R1 N. benthamiana plants transformed with GFP gene fused to N gene segments
| Gene | Line no. | No. of plants tested | Reactions of test plants
|
||
|---|---|---|---|---|---|
| HS | HT | HR | |||
| GFP + 5/8 N | 1 | 18 | 10 | 7 | 1 |
| 3 | 14 | 14 | |||
| GFP + 3/4 N | 5 | 17 | 8 | 9 | |
| 6 | 18 | 6 | 12 | ||
| 7 | 18 | 15 | 3 | ||
| 22 | 20 | 20 | |||
| 23 | 20 | 16 | 1 | 3 | |
| 24 | 16 | 16 | |||
| GFP + 2/2 N | 8 | 15 | 9 | 1 | 5 |
| 9 | 18 | 18 | |||
| 10 | 16 | 16 | |||
| 26 | 22 | 19 | 3 | ||
| 27 | 19 | 19 | |||
| 28 | 21 | 18 | 1 | 2 | |
| 29 | 21 | 1 | 20 | ||
| Control | 64 | 64 | |||
Figure 5.
Northern analysis of R1 plants containing the GFP/N gene fusions. Leaves were harvested for RNA isolation when the plants were at the 5- to 6-leaf stage. Ten micrograms per lane of total RNAs was used for Northern blots, which were probed with the N gene of TSWV-BL (Upper) or the GFP gene (Lower). After removal of leaf samples for RNA analysis, the plants were inoculated with TSWV-BL and rated as either resistant (R) or susceptible (S), depending on the presence or absence of systemic symptoms at 14 days after inoculation. Lanes 1 and 2, GFP + 5/8 N line 1 R1 plants; lanes 3–6, GFP + 3/4 N line 5 R1 plants; lane 7, GFP + 3/4 N line 6 R1 plants; lane 8, GFP + 3/4 N line 23 R1 plants; lanes 9, 10, and 12, GFP + 2/2 N line 8 R1 plants; and lane 11, a nontransformed plant. Transcripts for GFP linked to 5/8 N, to 3/4 N, or to 2/2 N are ≈1110 bp, ≈1220bp, and ≈1450 bp, respectively.
DISCUSSION
We have generated a number of plant lines that express different regions of TSWV N gene alone or fused with a nonviral sequence, GFP. Our studies have shown that transgenes smaller than one-quarter (235 bp) of the N gene were ineffective when expressed alone but were effective when fused to the GFP gene for post-transcriptional inactivation of the homologous, incoming, tospovirus. This result suggests that the inability of the small N transgenes alone to induce homology-dependent virus resistance was due not to their insufficient lengths of homology to the silenced transgene (in this case the virus genome) but because they are incapable of inducing gene silencing. Thus, this study differentiates the ability to induce transgene silencing from the ability to provide homology-dependent trans-inactivation.
Inability of the small N gene segments to induce transgene silencing can be due to their inefficient transcription in nuclei, inefficient processing/transport, and/or less stability of their transcripts in the cytoplasm. This conclusion is consistent with our Northern blot analysis, showing that the transcript of the small transgenes accumulated at much lower levels in the cytoplasm than the level of the large transgenes (Fig. 4). The reduced transcript accumulation of the small transgenes may result from steric interference of transcription initiation machinery with the transcription termination machinery. Alternatively, the small N gene transcripts may lose critical RNA sequence elements required for effective mRNA processing, transport, and/or stability, resulting in much less mature mRNA accumulation in the cytoplasm (45). The addition of the GFP gene to the small transgenes may simply enhance their ability to transcribe the transgene fusions, or the GFP gene may provide RNA sequence elements required for accumulation of the mature mRNA.
On the other hand, trans-inactivation of the silenced transgene requires much shorter sequence of homology. In this case, when expressed as a fusion with GFP, transgenes as short as 110 bp can trans-inactivate the incoming virus genome. This short homologous sequence presumably interacts with the incoming virus to form RNA duplex, which serves as a target for cellular degradation. The observation that nearly all of the transgenic plants with the smallest fusion transgene, GFP–5/8 N, were susceptible to TSWV (Tables 3 and 4) indicates that 110 bp (1/8 N) sequence of homology may be approaching the minimal length of homology required for trans-inactivation of the silenced genes (in this case the virus genome). This result is consistent with the recent observation of Sijen et al. (21). They showed that a small homologous sequence of only 60 nucleotides was sufficient to tag a recombinant potato virus X molecule for the gene-silencing-mediated elimination process. As we reported here, they also showed that the frequency and quality of resistance appeared to depend on both the length of the homologous sequence and the concentration of the inoculum.
Our study also shows that any half of the N gene (first, middle, or second) can confer post-transcriptional gene silencing-derived viral resistance (Table 2). This result suggests that the specific RNA secondary structure of the N gene sequence might not be necessary for inducing transgene silencing and viral resistance. Small segments (110–235 bp) were ineffective when expressed alone but were effective when fused to the GFP gene for post-transcriptional gene silencing and viral resistance. Taken together, the results indicated that the post-transcriptional gene silencing-derived virus resistance is dependent on the transgene length, but not on the specific sequences within the N gene of TSWV. These results also provide us the hint that any small part of the viral genome larger than a certain length might confer resistance when fused with a silencer DNA (e.g., GFP). Thus, we suggest that any viral sequence longer than 110 bp could confer RNA-mediated resistance when fused to stably expressed normal-length nonviral transgene. If it is true, it would significantly facilitate the engineering of viral resistance because isolation of a specific viral gene such as coat protein gene or replicase gene can be very tedious, especially if the viral genome organization is not well characterized. It should be pointed out that the coat protein gene will continue to be one of the best choices for RNA-mediated resistance because it is highly expressed and its transcript is presumably very stable in the infected cells.
Post-transcription gene silencing-derived resistance is an effective way for developing virus resistant transgenic plants. Furthermore, there are several advantages for inducing the gene silencing state with a chimeric transgene consisting of a silencer DNA (e.g., GFP) fused with one or more small nontranslatable segments of a viral genome(s). First, the silencer DNA can enhance the induction of gene silencing. Second, linking several segments of the viral genes could provide multiple virus resistance. Third, nontranslatable construction produces no protein, thus reducing the possible complementation of naturally occurring mutants and transencapsidation of other viruses. And fourth, the small segment also reduces the possibility of recombination with other viral genomes.
Acknowledgments
We thank Sheri Day and David Hummer for technical assistance. This work was supported in part by a grant from Asgrow Seed Company.
ABBREVIATIONS
- TSWV
tomato spotted wilt virus
- N
nucleocapsid protein
- GFP
green fluorescent protein
- INSV
impatiens necrotic spot virus
- GRSV
groundnut ringspot virus
References
- 1.van der Krol A R, Mur L A, Beld M, Mol J N M, Stuitje A R. Plant Cell. 1990;2:291–300. doi: 10.1105/tpc.2.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Napoli C, Lemieux C, Jorgensen R. Plant Cell. 1990;2:279–290. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Carvalho F, Gheysen G, Kushnir S, Van Montagu M, Inze D, Castresana C. EMBO J. 1992;11:2595–2602. doi: 10.1002/j.1460-2075.1992.tb05324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linn F, Heidmann I, Saedler H, Meyer P. Mol Gen Genet. 1990;222:329–336. doi: 10.1007/BF00633837. [DOI] [PubMed] [Google Scholar]
- 5.Scheid O M, Paszkowski J, Potrykus I. Mol Gen Genet. 1991;228:104–112. doi: 10.1007/BF00282454. [DOI] [PubMed] [Google Scholar]
- 6.Hobbs S L A, Kpodar P, DeLong C M O. Plant Mol Biol. 1990;15:851–864. doi: 10.1007/BF00039425. [DOI] [PubMed] [Google Scholar]
- 7.Hobbs S L A, Warkentin T D, DeLong C M O. Plant Mol Biol. 1993;21:17–26. doi: 10.1007/BF00039614. [DOI] [PubMed] [Google Scholar]
- 8.Hart C M, Fischer B, Neuhaus J M, Meins F J. Mol Gen Genet. 1992;235:179–188. doi: 10.1007/BF00279359. [DOI] [PubMed] [Google Scholar]
- 9.Assaad F F, Tucker K L, Signer E R. Plant Mol Biol. 1993;22:1067–1085. doi: 10.1007/BF00028978. [DOI] [PubMed] [Google Scholar]
- 10.Vaucheret H, Palauqui J C, Elmayan T, Moffatt B. Mol Gen Genet. 1995;248:311–317. doi: 10.1007/BF02191598. [DOI] [PubMed] [Google Scholar]
- 11.Finnegan J, McElroy D. Bio/Technology. 1994;12:883–888. [Google Scholar]
- 12.Matzke M A, Matzke A J M. Plant Physiol. 1995;107:679–685. doi: 10.1104/pp.107.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorgensen R A. Science. 1995;268:686–691. doi: 10.1126/science.268.5211.686. [DOI] [PubMed] [Google Scholar]
- 14.Meyer P, Saedler H. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:23–48. doi: 10.1146/annurev.arplant.47.1.23. [DOI] [PubMed] [Google Scholar]
- 15.Van Blokland R, Van der Greest N, Mol J N M, Kooter J M. Plant J. 1994;6:861–877. [Google Scholar]
- 16.Meyer P, Heidmann I, Niedenhof I. Plant J. 1993;4:86–100. doi: 10.1046/j.1365-313x.1993.04010089.x. [DOI] [PubMed] [Google Scholar]
- 17.Neuhuber F, Park Y D, Matzke A J M, Matzke M A. Mol Gen Genet. 1994;244:230–241. doi: 10.1007/BF00285450. [DOI] [PubMed] [Google Scholar]
- 18.Brusslan J A, Karlin N G A, Huang L, Tobin E M. Plant Cell. 1993;5:667–677. doi: 10.1105/tpc.5.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingelbrecht I, Van Houdt H, Van Montagu M, Depicker A. Proc Natl Acad Sci USA. 1994;91:10502–10506. doi: 10.1073/pnas.91.22.10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith H A, Swaney S L, Parks T D, Wernsman E A, Dougherty W G. Plant Cell. 1994;6:1441–1453. doi: 10.1105/tpc.6.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sijen T, Wellink J, Hiriart J-B, van Kammen A. Plant Cell. 1996;8:2227–2294. doi: 10.1105/tpc.8.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.English J J, Mueller E, Baulcombe D C. Plant Cell. 1996;8:179–188. doi: 10.1105/tpc.8.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodwin J, Chapman K, Swaney S, Parks T D, Wernsman E A, Dougherty W G. Plant Cell. 1996;8:95–105. doi: 10.1105/tpc.8.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindbo J A, Silva-Rosales L, Proebsting W M, Dougherty W G. Plant Cell. 1993;5:1749–1759. doi: 10.1105/tpc.5.12.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller E, Gilbert J, Davenport G, Brigneti G, Baulcombe D C. Plant J. 1995;7:1001–1013. [Google Scholar]
- 26.Dawson W D. Trends Plant Sci. 1996;1:107–108. [Google Scholar]
- 27.Pang S Z, Jan F J, Carney K, Stout J, Tricoli D M, Quemada H D, Gonsalves D. Plant J. 1996;9:899–909. [Google Scholar]
- 28.Prins M, Resende R D O, Anker C, van Schepen A, de Haan P, Goldbach R. Mol Plant–Microbe Interact. 1996;9:416–418. doi: 10.1094/mpmi-9-0416. [DOI] [PubMed] [Google Scholar]
- 29.Baulcombe D C. Plant Cell. 1996;8:1833–1844. doi: 10.1105/tpc.8.10.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dougherty W G, Parks T D. Curr Opin Cell Biol. 1995;7:399–405. doi: 10.1016/0955-0674(95)80096-4. [DOI] [PubMed] [Google Scholar]
- 31.Jorgensen R. AgBiotech News Inform. 1992;4:265N–273N. [Google Scholar]
- 32.Baulcombe D C, English J J. Curr Opin Biotechnol. 1996;7:173–180. [Google Scholar]
- 33.Pang S-Z, Nagpala P, Wang M, Slightom J L, Gonsalves D. Phytopathology. 1992;82:1223–1229. [Google Scholar]
- 34.Pang S Z, Slightom J L, Gonsalves D. Bio/Technology. 1993;11:819–824. doi: 10.1038/nbt0793-819. [DOI] [PubMed] [Google Scholar]
- 35.Pang S-Z, Bock J H, Gonsalves C, Slightom J L, Gonsalves D. Phytopathology. 1994;84:243–249. [Google Scholar]
- 36.de Haan P, Wagemakers L, Peters D, Goldbach R. J Gen Virol. 1990;71:1001–1008. doi: 10.1099/0022-1317-71-5-1001. [DOI] [PubMed] [Google Scholar]
- 37.Kormelink R, de Haan P, Meurs C, Peters D, Goldbach R. J Gen Virol. 1992;73:2795–2804. doi: 10.1099/0022-1317-73-11-2795. [DOI] [PubMed] [Google Scholar]
- 38.Law M D, Speck J, Moyer J W. J Gen Virol. 1991;72:2597–2602. doi: 10.1099/0022-1317-72-10-2597. [DOI] [PubMed] [Google Scholar]
- 39.Law M D, Speck J, Moyer J W. Virology. 1992;188:732–741. doi: 10.1016/0042-6822(92)90528-w. [DOI] [PubMed] [Google Scholar]
- 40.Maiss E, Ivanova L, Breyel E, Adam G. J Gen Virol. 1991;72:461–464. doi: 10.1099/0022-1317-72-2-461. [DOI] [PubMed] [Google Scholar]
- 41.Pang S-Z, Slightom J L, Gonsalves D. Phytopathology. 1993;83:728–733. [Google Scholar]
- 42.Kormelink R, Van Poelwijk F, Peters D, Goldbach R. J Gen Virol. 1992;73:2125–2128. doi: 10.1099/0022-1317-73-8-2125. [DOI] [PubMed] [Google Scholar]
- 43.de Haan P, Kormelink R, Resende R D, Van Poelwijk F, Peters D, Goldbach R. J Gen Virol. 1991;72:2207–2216. doi: 10.1099/0022-1317-72-9-2207. [DOI] [PubMed] [Google Scholar]
- 44.Horsch R B, Fry J E, Hoffman N L, Eichholtz D, Rogers S G, Fraley R T. Science. 1985;227:1229–1231. [Google Scholar]
- 45.Caponigro G, Parker R. Microbiol Rev. 1996;60:233–249. doi: 10.1128/mr.60.1.233-249.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]