Abstract
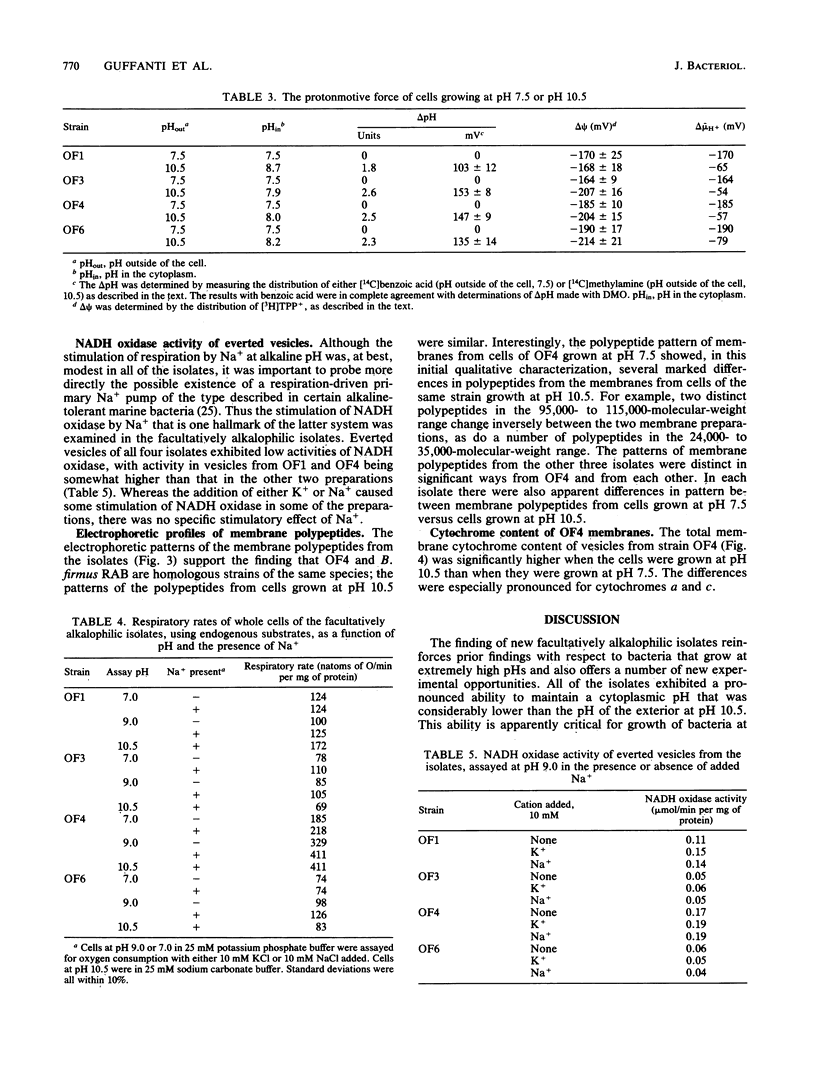
Four facultatively alkalophilic isolates were purified from enrichment cultures initiated with lime-treated garden soil. Four isolates, OF1, OF3, OF4, and OF6, were obligately aerobic, spore-forming, gram-positive, motile rods which were capable of growth at both pH 7.5 and pH 10.5. Strains OF1 and OF6 grew best at the lower pH value; and whereas growth of these strains at pH 10.5 was completely dependent on added Na+, growth at pH 7.5 was only partially dependent on added Na+. Strains OF3 and OF4 grew better at pH 10.5 than at pH 7.5, with strain OF3 growing modestly over its entire pH range, while OF4 grew well. Growth of OF3 and OF4 was completely dependent on added Na+ at both pH 7.5 and pH 10.5. DNA-DNA hybridization studies indicated that OF1 and OF6 are closely related strains but are not related to the other isolates, Bacillus subtilis, or two previously studied obligately alkalophilic bacilli. OF3 was unrelated to any of the other organisms examined in the study, whereas OF4 showed complete homology with obligately alkalophilic Bacillus firmus RAB. All four isolates maintained a cytoplasmic pH that was considerably lower than the external pH when the latter was 10.5. Although substantial transmembrane electrical potentials were observed, the total electrochemical proton gradient (delta mu H+) was low at pH 10.5 in all the strains. By contrast, delta mu H+ was substantial at pH 7.5 and at that pH was composed entirely of an electrical potential. These results are in contrast to previous findings that obligately alkalophilic bacilli generate only small electrical potentials at near neutral pH. All the isolates exhibited substantial rates of respiration as measured by oxygen consumption. Neither respiration nor NADH oxidation by everted membrane vesicles was significantly stimulated by Na+. Analyses of reduced versus oxidized difference spectra of membranes from OF4 showed that the total membrane cytochrome content was considerably higher in cells grown at pH 10.5 than at pH 7.5, with the levels of c- and a-type cytochromes exhibiting the largest pH-dependent differences. Initial examination of membrane protein profiles on gel electrophoresis also indicated a number of changes in pattern in each isolate, depending on the growth pH.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Guffanti A. A., Susman P., Blanco R., Krulwich T. A. The protonmotive force and alpha-aminoisobutyric acid transport in an obligately alkalophilic bacterium. J Biol Chem. 1978 Feb 10;253(3):708–715. [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Kambe T., Kagawa Y. A purified alanine carrier composed of a single polypeptide from thermophilic bacterium PS3 driven by either proton or sodium ion gradient. J Biol Chem. 1984 Sep 10;259(17):10653–10656. [PubMed] [Google Scholar]
- Kitada M., Guffanti A. A., Krulwich T. A. Bioenergetic properties and viability of alkalophilic Bacillus firmus RAB as a function of pH and Na+ contents of the incubation medium. J Bacteriol. 1982 Dec;152(3):1096–1104. doi: 10.1128/jb.152.3.1096-1104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Agus R., Schneier M., Guffanti A. A. Buffering capacity of bacilli that grow at different pH ranges. J Bacteriol. 1985 May;162(2):768–772. doi: 10.1128/jb.162.2.768-772.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Guffanti A. A., Fong M. Y., Falk L., Hicks D. B. Alkalophilic Bacillus firmus RAB generates variants which can grow at lower Na+ concentrations than the parental strain. J Bacteriol. 1986 Mar;165(3):884–889. doi: 10.1128/jb.165.3.884-889.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Guffanti A. A. Physiology of acidophilic and alkalophilic bacteria. Adv Microb Physiol. 1983;24:173–214. doi: 10.1016/s0065-2911(08)60386-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis R. J., Belkina S., Krulwich T. A. Alkalophiles have much higher cytochrome contents than conventional bacteria and than their own non-alkalophilic mutant derivatives. Biochem Biophys Res Commun. 1980 Jul 31;95(2):857–863. doi: 10.1016/0006-291x(80)90866-9. [DOI] [PubMed] [Google Scholar]
- Lewis R. J., Krulwich T. A., Reynafarje B., Lehninger A. L. Respiration-dependent proton translocation in alkalophilic Bacillus firmus RAB and its non-alkalophilic mutant derivative. J Biol Chem. 1983 Feb 25;258(4):2109–2111. [PubMed] [Google Scholar]
- Scher B. M., Dean D. H., Garro A. J. Fragmentation of Bacillus bacteriophage phi105 DNA by complementary single-stranded DNA in the cohesive ends of the molecule. J Virol. 1977 Aug;23(2):377–383. doi: 10.1128/jvi.23.2.377-383.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S., Kaback H. R. Membrane potential and active transport in membrane vesicles from Escherichia coli. Biochemistry. 1975 Dec 16;14(25):5451–5461. doi: 10.1021/bi00696a011. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H., Unemoto T. Na+ is translocated at NADH:quinone oxidoreductase segment in the respiratory chain of Vibrio alginolyticus. J Biol Chem. 1984 Jun 25;259(12):7785–7790. [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Agmon V., Schuldiner S., Padan E. The sodium/proton antiporter is part of the pH homeostasis mechanism in Escherichia coli. J Biol Chem. 1982 Apr 10;257(7):3687–3691. [PubMed] [Google Scholar]




