Abstract
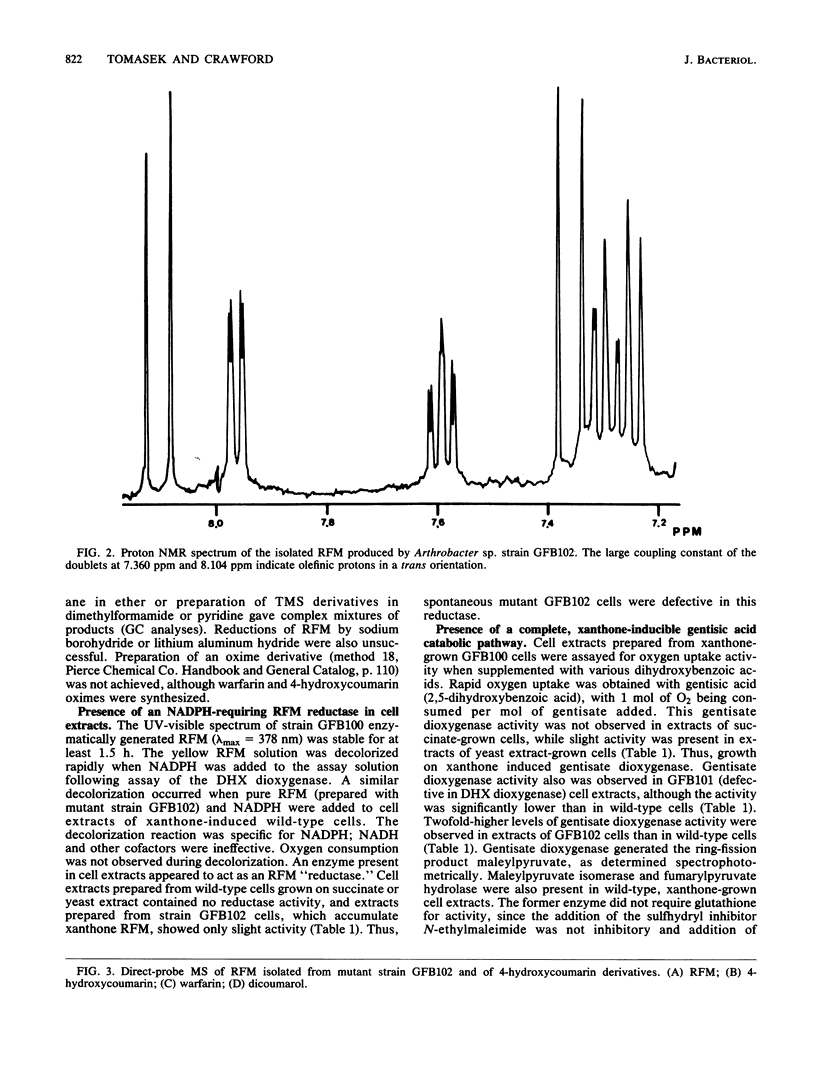
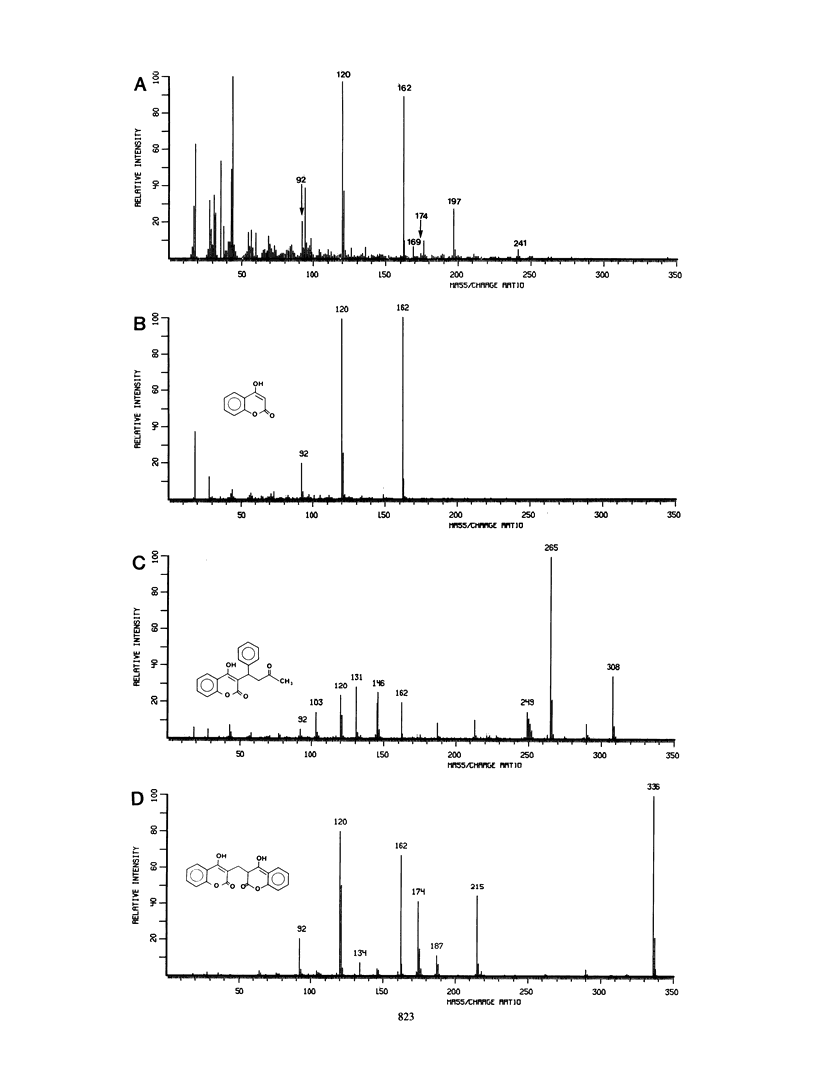
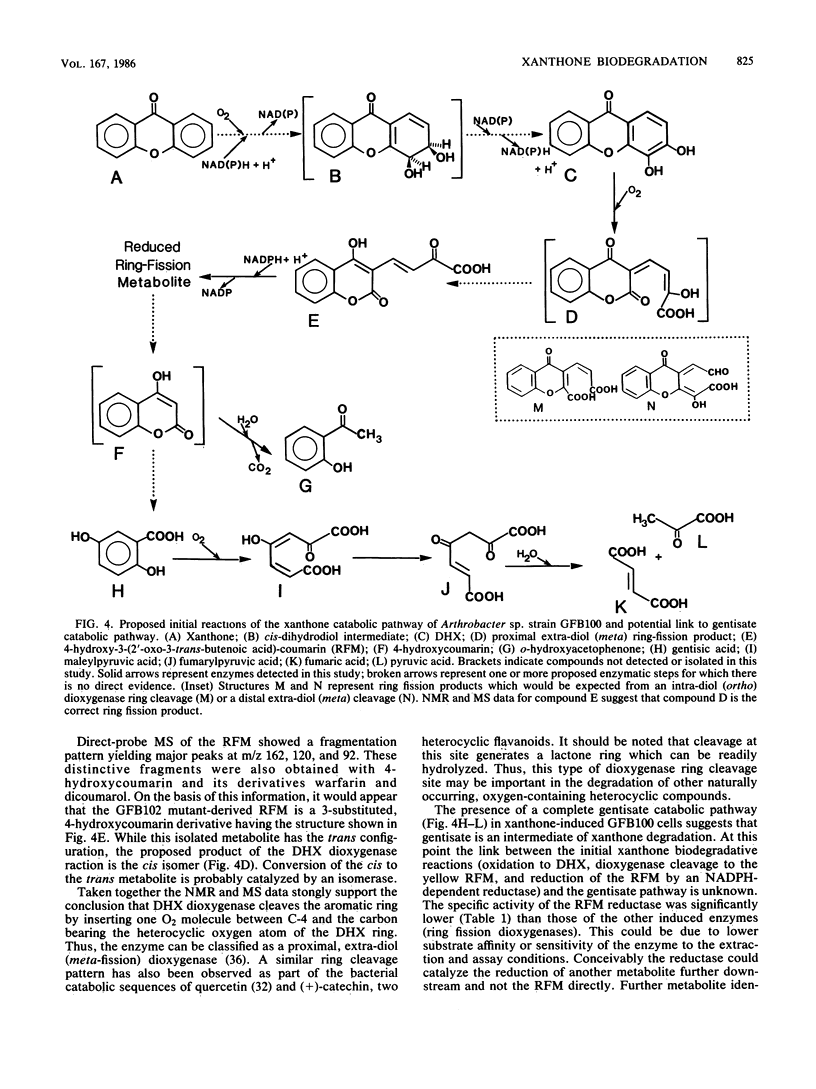
This study examined the catabolism of xanthone by an Arthrobacter sp. (strain GFB100) capable of growth on xanthone as its main source of carbon and energy. An early catabolic intermediate was 3,4-dihydroxyxanthone. This compound was isolated from the growth medium of a mutant strain of the Arthrobacter sp. which lacked the xanthone-inducible dihydroxyxanthone ring-fission dioxygenase of the wild-type strain. Cell extracts from wild-type xanthone-grown cells oxidized 3,4-dihydroxyxanthone to a yellow ring-fission metabolite. The same yellow compound accumulated in xanthone-grown cultures of a spontaneous mutant which lacked an active, xanthone-inducible, NADPH-linked ring-fission metabolite reductase. The yellow ring-fission metabolite appears to be 4-hydroxy-3-(2'-oxo-3-trans-butenoate)-coumarin, based on its nuclear magnetic resonance spectrum and mass spectral fragmentation pattern, indicating that ring cleavage of 3,4-dihydroxyxanthone was by an extra-diol (meta-fission) mechanism. Enzymatic analyses indicated that growth on xanthone induced a complete gentisate pathway: dioxygenase-catalyzed cleavage of gentisate to maleylpyruvate, isomerization of maleylpyruvate to fumarylpyruvate, and hydrolysis of fumarylpyruvate to fumarate and pyruvate. 4-Hydroxycoumarin was thought to be a likely pathway intermediate linking the early xanthone catabolic steps to the gentisate pathway, since 2-hydroxyacetophenone, a byproduct of 4-hydroxycoumarin hydrolysis, was formed when wild-type cells were cultured with xanthone. Chlorinated 2-hydroxyacetophenones were also obtained from specific chloro-substituted xanthones.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnsley E. A. Bacterial oxidation of naphthalene and phenanthrene. J Bacteriol. 1983 Feb;153(2):1069–1071. doi: 10.1128/jb.153.2.1069-1071.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E., Morgan J. C., Gibson D. T. Bacterial and fungal oxidation of dibenzofuran. Biochem J. 1979 Apr 15;180(1):175–185. doi: 10.1042/bj1800175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chexal K. K., Holker J. S., Simpson T. J., Young K. The biosynthesis of fungal metabolites. Part V. Structure of variecoxanthones A, B, and C, metabolites of Aspergillus variecolor; conversion of variecoxanthone a into (plus or minus)-de-c-prenylepishamixanthone. J Chem Soc Perkin 1. 1975;(6):543–548. [PubMed] [Google Scholar]
- Crawford R. L., Frick T. D. Rapid spectrophotometric differentiation between glutathione-dependent and glutathione-independent gentisate and homogentisate pathways. Appl Environ Microbiol. 1977 Aug;34(2):170–174. doi: 10.1128/aem.34.2.170-174.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L. Novel pathway for degradation of protocatechuic acid in Bacillus species. J Bacteriol. 1975 Feb;121(2):531–536. doi: 10.1128/jb.121.2.531-536.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L., Olson P. E., Frick T. D. Catabolism of 5-chlorosalicylate by a Bacillus isolated from the Mississippi River. Appl Environ Microbiol. 1979 Sep;38(3):379–384. doi: 10.1128/aem.38.3.379-384.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps R. E., Trudgill P. W., Whateley J. G. The metabolism of 1-phenylethanol and acetophenone by Nocardia T5 and an Arthrobacter species. Eur J Biochem. 1978 May;86(1):175–186. doi: 10.1111/j.1432-1033.1978.tb12297.x. [DOI] [PubMed] [Google Scholar]
- Dagley S. A biochemical approach to some problems of environmental pollution. Essays Biochem. 1975;11:81–138. [PubMed] [Google Scholar]
- EVANS W. C., FERNLEY H. N., GRIFFITHS E. OXIDATIVE METABOLISM OF PHENANTHRENE AND ANTHRACENE BY SOIL PSEUDOMONADS. THE RING-FISSION MECHANISM. Biochem J. 1965 Jun;95:819–831. doi: 10.1042/bj0950819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. T., Roberts R. L., Wells M. C., Kobal V. M. Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun. 1973 Jan 23;50(2):211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- Hagedorn S. R., Bradley G., Chapman P. J. Glutathione-independent isomerization of maleylpyruvate by Bacillus megaterium and other gram-positive bacteria. J Bacteriol. 1985 Aug;163(2):640–647. doi: 10.1128/jb.163.2.640-647.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keddie N. C., Taylor A. W., Sykes P. A. The termination of the common bile duct. Br J Surg. 1974 Aug;61(8):623–625. doi: 10.1002/bjs.1800610808. [DOI] [PubMed] [Google Scholar]
- Kersten P. J., Dagley S., Whittaker J. W., Arciero D. M., Lipscomb J. D. 2-pyrone-4,6-dicarboxylic acid, a catabolite of gallic acids in Pseudomonas species. J Bacteriol. 1982 Dec;152(3):1154–1162. doi: 10.1128/jb.152.3.1154-1162.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klecka G. M., Gibson D. T. Metabolism of dibenzo[1,4]dioxan by a Pseudomonas species. Biochem J. 1979 Jun 15;180(3):639–645. doi: 10.1042/bj1800639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACK L. Enzymic cis-trans isomerization of maleylpyruvic acid. J Biol Chem. 1961 Nov;236:2835–2840. [PubMed] [Google Scholar]
- Laborde A. L., Gibson D. T. Metabolism of dibenzothiophene by a Beijerinckia species. Appl Environ Microbiol. 1977 Dec;34(6):783–790. doi: 10.1128/aem.34.6.783-790.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz E., Engle F. E., Wood J. M. New oxygenases in the degradation of flavones and flavanones by Pseudomonas putida. Biochemistry. 1974 Apr 9;13(8):1768–1776. doi: 10.1021/bi00705a033. [DOI] [PubMed] [Google Scholar]
- Stevenson I. L. Utilization of aromatic hydrocarbons by Arthrobacter spp. Can J Microbiol. 1967 Feb;13(2):205–211. doi: 10.1139/m67-027. [DOI] [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Microcultural study of bacterial size changes and microcolony and ultramicrocolony formation by heterotrophic bacteria in seawater. Appl Environ Microbiol. 1981 Feb;41(2):518–527. doi: 10.1128/aem.41.2.518-527.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



