Abstract
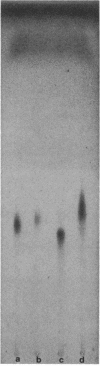
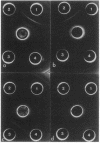
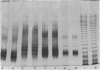
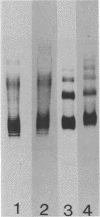
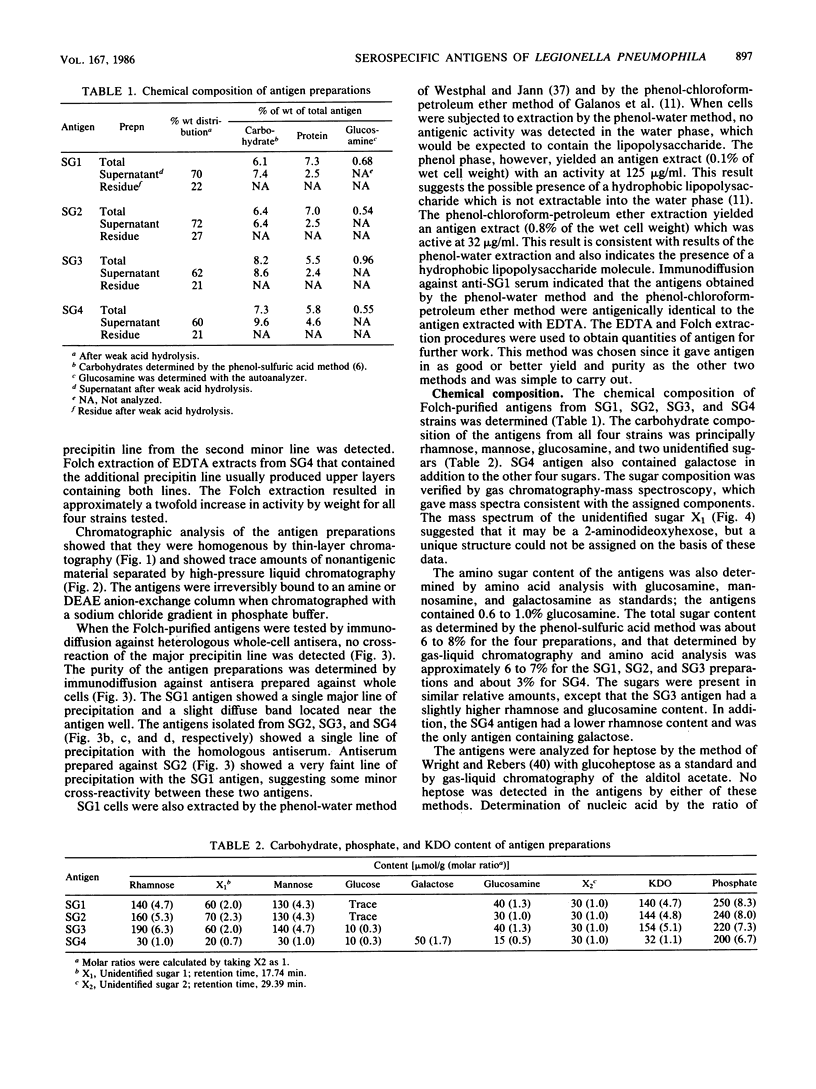
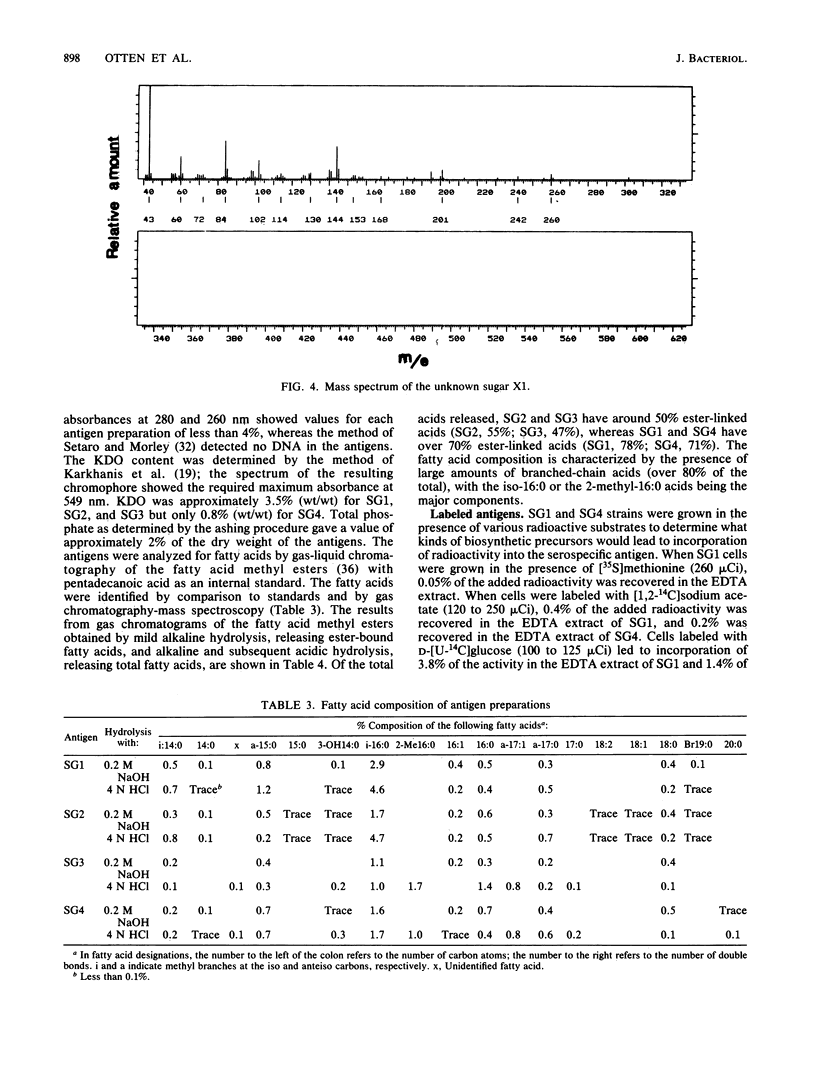
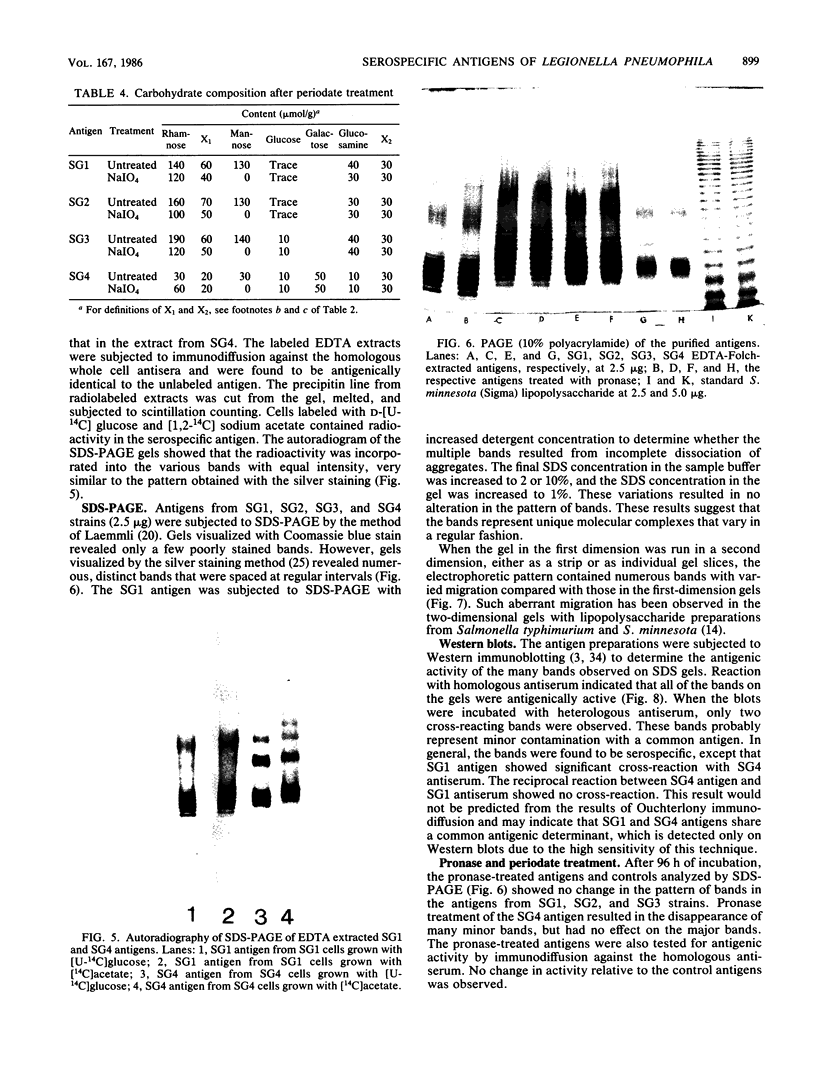
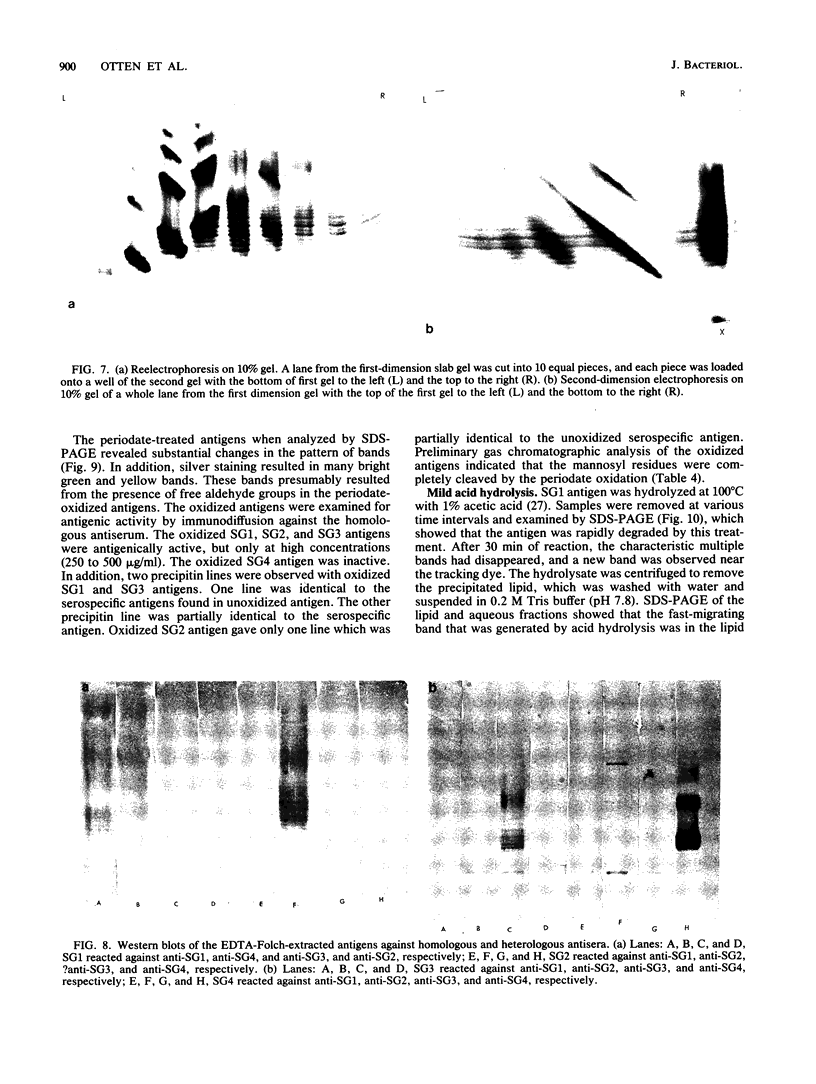
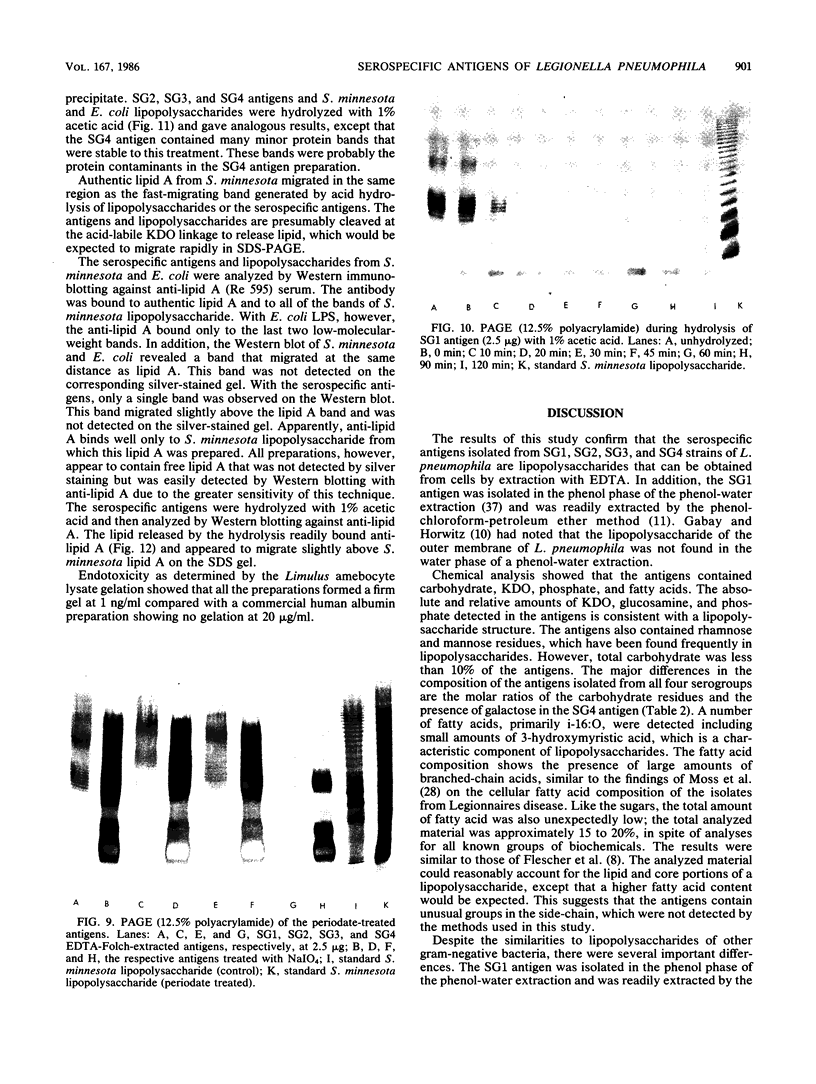
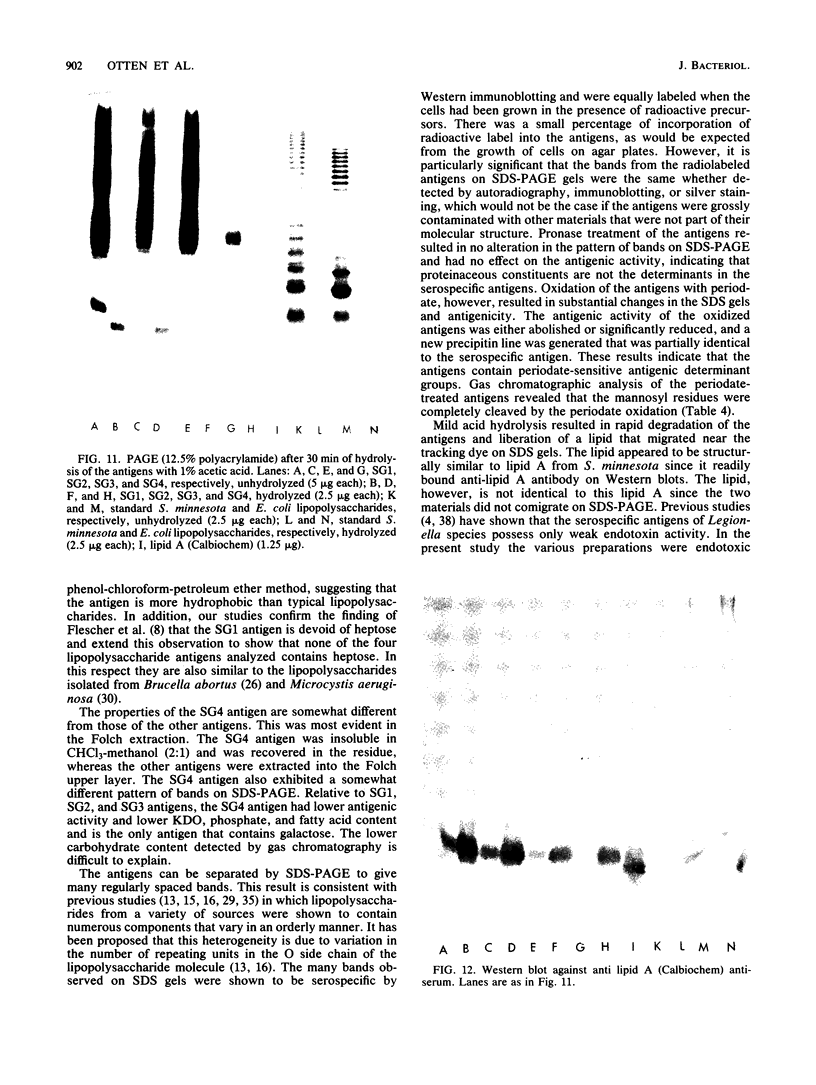
Serospecific antigens isolated by EDTA extraction from four serogroups of Legionella pneumophila were analyzed for their chemical composition, molecular heterogeneity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunological properties. The antigens were shown to be lipopolysaccharides and to differ from the lipopolysaccharides of other gram-negative bacteria. The serospecific antigens contained rhamnose, mannose, glucosamine, and two unidentified sugars together with 2-keto-3-deoxyoctonate, phosphate, and fatty acids. The fatty acid composition was predominantly branched-chain acids with smaller amounts of 3-hydroxymyristic acid. The antigens contain periodate-sensitive groups; mannosyl residues were completely cleaved by periodate oxidation. Hydrolysis of the total lipopolysaccharide by acetic acid resulted in the separation of a lipid A-like material that cross-reacted with the antiserum to lipid A from Salmonella minnesota but did not comigrate with it on sodium dodecyl sulfate gels. None of the four antigens contained heptose. All of the antigen preparations showed endotoxicity when tested by the Limulus amebocyte lysate assay. The results of this study indicate that the serogroup-specific antigens of L. pneumophila are lipopolysaccharides containing an unusual lipid A and core structure and different from those of other gram-negative bacteria.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chen C. L., Nowotny A. Direct determination of molar ratios of various chemical constituents in endotoxic glycolipids in silicic acid scrapings from thin-layer chromatographic plates. J Chromatogr. 1974 Oct 9;97(1):39–45. doi: 10.1016/s0021-9673(01)97582-x. [DOI] [PubMed] [Google Scholar]
- Collins M. T., Espersen F., Høiby N., Cho S. N., Friis-Møller A., Reif J. S. Cross-reactions between Legionella pneumophila (serogroup 1) and twenty-eight other bacterial species, including other members of the family Legionellaceae. Infect Immun. 1983 Mar;39(3):1441–1456. doi: 10.1128/iai.39.3.1441-1456.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Feeley J. C., Gibson R. J., Gorman G. W., Langford N. C., Rasheed J. K., Mackel D. C., Baine W. B. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979 Oct;10(4):437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesher A. R., Jennings H. J., Lugowski C., Kasper D. L. Isolation of a serogroup 1-specific antigen from Legionella pneumophila. J Infect Dis. 1982 Feb;145(2):224–233. doi: 10.1093/infdis/145.2.224. [DOI] [PubMed] [Google Scholar]
- Gabay J. E., Horwitz M. A. Isolation and characterization of the cytoplasmic and outer membranes of the Legionnaires' disease bacterium (Legionella pneumophila). J Exp Med. 1985 Feb 1;161(2):409–422. doi: 10.1084/jem.161.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of antisera against the lipid-A component of bacterial lipopolysaccharides. Eur J Biochem. 1971 Dec 22;24(1):116–122. doi: 10.1111/j.1432-1033.1971.tb19661.x. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J. Aberrant migration of lipopolysaccharide in sodium dodecyl sulfate/polyacrylamide gel electrophoresis. Eur J Biochem. 1983 Jul 1;133(3):685–688. doi: 10.1111/j.1432-1033.1983.tb07517.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann B., Reske K., Jann K. Heterogeneity of lipopolysaccharides. Analysis of polysaccharide chain lengths by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Eur J Biochem. 1975 Dec 1;60(1):239–246. doi: 10.1111/j.1432-1033.1975.tb20996.x. [DOI] [PubMed] [Google Scholar]
- Johnson W., Elliott J. A., Helms C. M., Renner E. D. A high molecular weight antigen in Legionnaires' disease bacterium: isolation and partial characterization. Ann Intern Med. 1979 Apr;90(4):638–641. doi: 10.7326/0003-4819-90-4-638. [DOI] [PubMed] [Google Scholar]
- Johnson W., Pesanti E., Elliott J. Serospecificity and opsonic activity of antisera to Legionella pneumophila. Infect Immun. 1979 Nov;26(2):698–704. doi: 10.1128/iai.26.2.698-704.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKinney R. M., Thomason B. M., Harris P. P., Thacker L., Lewallen K. R., Wilkinson H. W., Hebert G. A., Moss C. W. Recognition of a new serogroup of Legionnaires disease bacterium. J Clin Microbiol. 1979 Jan;9(1):103–107. doi: 10.1128/jcm.9.1.103-107.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Moreno E., Pitt M. W., Jones L. M., Schurig G. G., Berman D. T. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979 May;138(2):361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. T., Partridge S. M. Studies in immunochemistry: The use of phenol and of alkali in the degradation of antigenic material isolated from Bact. dysenteriae (Shiga). Biochem J. 1941 Nov;35(10-11):1140–1163. doi: 10.1042/bj0351140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Weaver R. E., Dees S. B., Cherry W. B. Cellular fatty acid composition of isolates from Legionnaires disease. J Clin Microbiol. 1977 Aug;6(2):140–143. doi: 10.1128/jcm.6.2.140-143.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Raziuddin S., Siegelman H. W., Tornabene T. G. Lipopolysaccharides of the cyanobacterium Microcystis aeruginosa. Eur J Biochem. 1983 Dec 1;137(1-2):333–336. doi: 10.1111/j.1432-1033.1983.tb07833.x. [DOI] [PubMed] [Google Scholar]
- Schramek S., Kazár J., Bazovská S. Lipid A in Legionella pneumophila. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Jul;252(3):401–404. [PubMed] [Google Scholar]
- Setaro F., Morley C. D. A rapid colorimetric assay for DNA. Anal Biochem. 1977 Aug;81(2):467–471. doi: 10.1016/0003-2697(77)90721-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wong K. H., Moss C. W., Hochstein D. H., Arko R. J., Schalla W. O. "Endotoxicity" of the Legionnaires' disease bacterium. Ann Intern Med. 1979 Apr;90(4):624–627. doi: 10.7326/0003-4819-90-4-624. [DOI] [PubMed] [Google Scholar]
- Wright B. G., Rebers P. A. Procedure for determining heptose and hexose in lipopolysaccharides. Modification of the cysteine-sulfuric acid method. Anal Biochem. 1972 Oct;49(2):307–319. doi: 10.1016/0003-2697(72)90433-2. [DOI] [PubMed] [Google Scholar]