Abstract
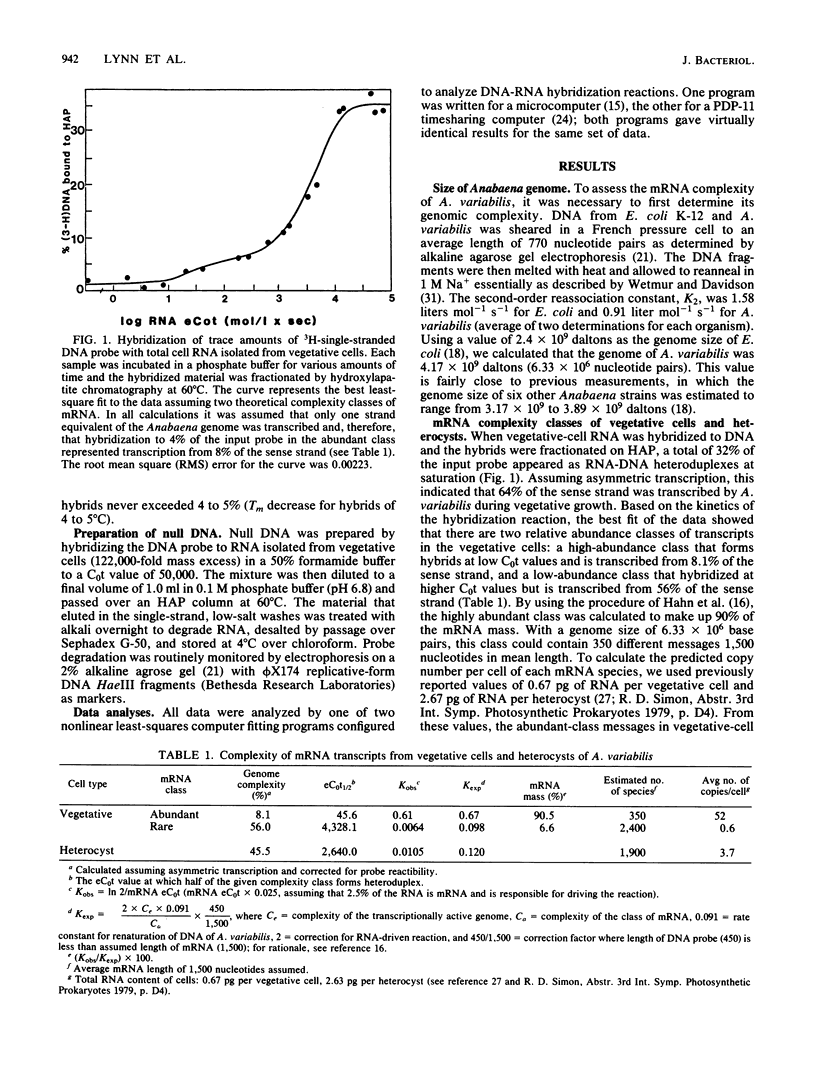
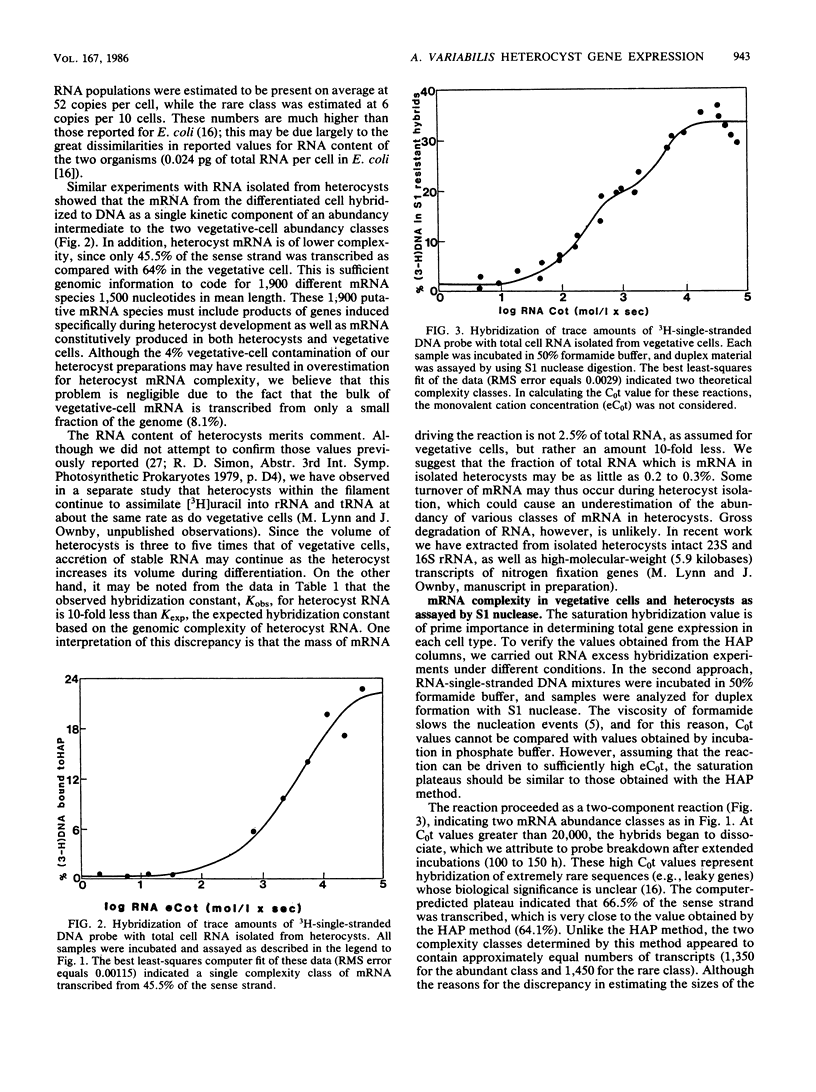
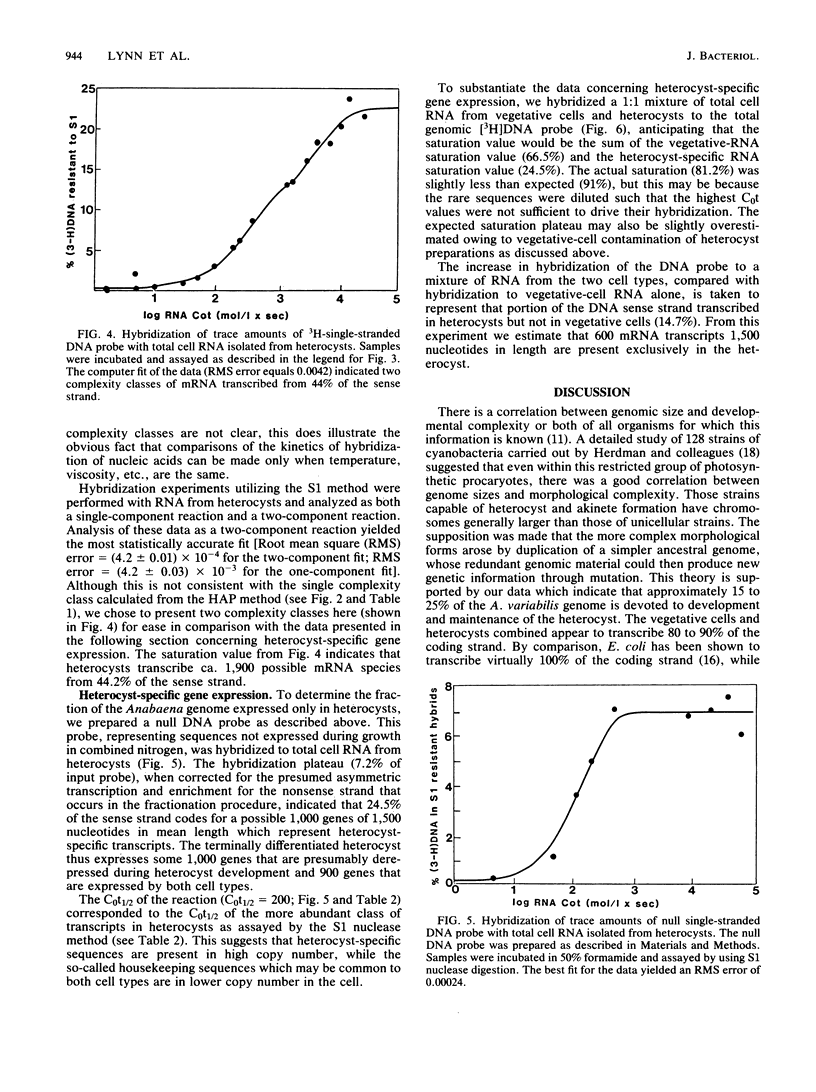
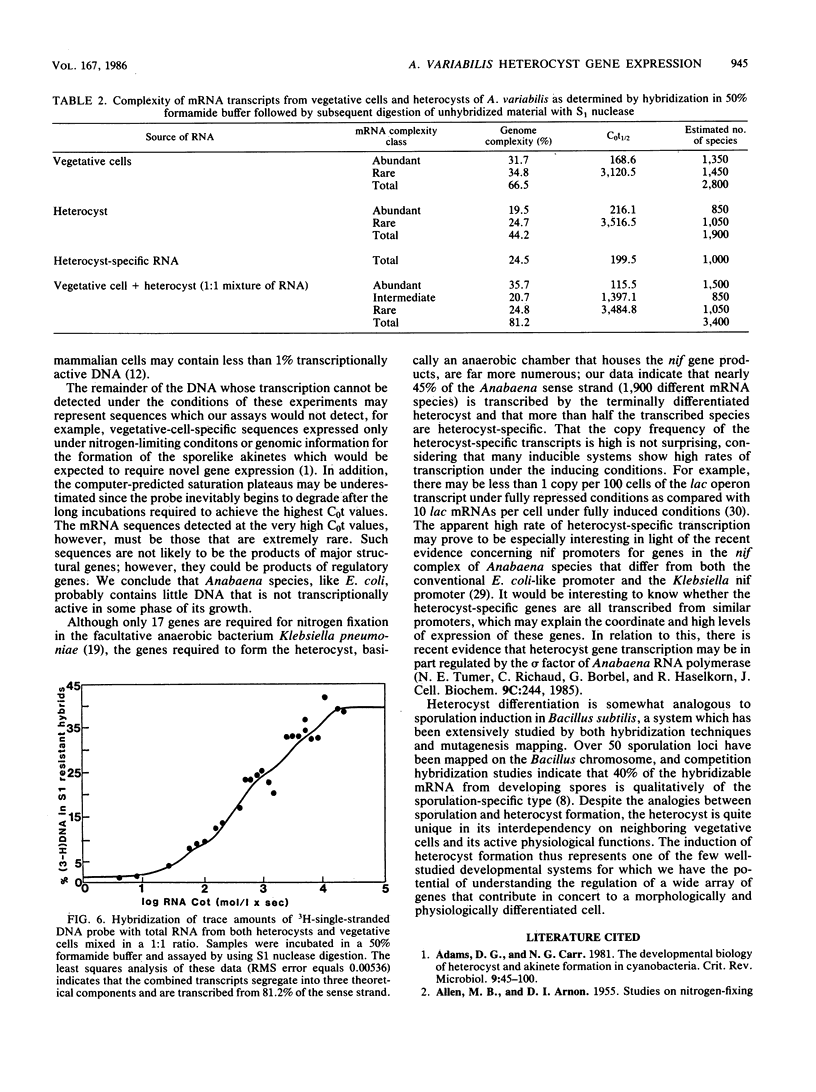
In the filamentous cyanobacterium Anabaena variabilis, specialized cells called heterocysts occur in a regular pattern along the filament and are the sites of nitrogen fixation. We used two different types of DNA-excess RNA hybridization techniques to estimate the number of genes expressed in recently differentiated, mature heterocysts. In the first, RNA and DNA were incubated in a phosphate buffer at 60 degrees C, and the hybrids were separated from the unhybridized material by hydroxylapatite chromatography. In the second, the nucleic acids were incubated at 50 degrees C in a buffer containing 50% formamide, and the fraction of DNA in duplexes was assayed by S1 nuclease digestion. Both techniques revealed that approximately 65% of the A. variabilis genome was expressed in vegetative cells and 45% of the genome was expressed in heterocysts. Two experiments were conducted to estimate the number of heterocyst-specific mRNA transcripts. In one, hybridization of heterocyst RNA to a null DNA probe (DNA not transcribed in vegetative cells) revealed that heterocyst-specific transcripts were encoded by 25% of the DNA sense strand, representing approximately 1,000 genes (assuming each to be 1,500 nucleotides in length). The second approach, in which total cell DNA was hybridized to a mixture of heterocyst and vegetative cell RNA, indicated that 14.7% of the DNA sense strand, or about 600 genes, was transcribed exclusively in the heterocyst. The remaining 900 to 1,300 transcripts present in the heterocyst appeared to be constitutively produced in both vegetative cells and heterocysts. The heterocyst-specific transcripts were present in abundant copies in the cell, while transcripts that occurred in both cell types were present at much lower frequency.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. G., Carr N. G. The developmental biology of heterocyst and akinete formation in cyanobacteria. Crit Rev Microbiol. 1981;9(1):45–100. doi: 10.3109/10408418109104486. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi R. H. Genetic control of sporulation. Annu Rev Genet. 1977;11:29–48. doi: 10.1146/annurev.ge.11.120177.000333. [DOI] [PubMed] [Google Scholar]
- Fleming H., Haselkorn R. Differentiation in Nostoc muscorum: nitrogenase is synthesized in heterocysts. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2727–2731. doi: 10.1073/pnas.70.10.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming H., Haselkorn R. The program of protein synthesis during heterocyst differentiation in nitrogen-fixing blue-green algae. Cell. 1974 Oct;3(2):169–170. doi: 10.1016/0092-8674(74)90121-4. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Britten R. J., Davidson E. H. A measurement of the sequence complexity of polysomal messenger RNA in sea urchin embryos. Cell. 1974 May;2(1):9–20. doi: 10.1016/0092-8674(74)90003-8. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Klein W. H., Davis M. M., Wold B. J., Britten R. J., Davidson E. H. Structural gene sets active in embryos and adult tissues of the sea urchin. Cell. 1976 Apr;7(4):487–505. doi: 10.1016/0092-8674(76)90200-2. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Robinson S. J., Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985 Apr 4;314(6010):419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- Green S., Field J. K., Green C. D., Beynon R. J. A microcomputer program for analysis of nucleic acid hybridization data. Nucleic Acids Res. 1982 Feb 25;10(4):1411–1420. doi: 10.1093/nar/10.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn W. E., Pettijohn D. E., Van Ness J. One strand equivalent of the Escherichia coli genome is transcribed: complexity and abundance classes of mRNA. Science. 1977 Aug 5;197(4303):582–585. doi: 10.1126/science.327551. [DOI] [PubMed] [Google Scholar]
- Kennedy C. Linkage map of the nitrogen fixation (nif) genes in Klebsiella pneumoniae. Mol Gen Genet. 1977 Nov 29;157(2):199–204. doi: 10.1007/BF00267398. [DOI] [PubMed] [Google Scholar]
- Martinson H. G. The nucleic acid-hydroxylapatite interaction. II. Phase transitions in the deoxyribonucleic acid-hydroxylapatite system. Biochemistry. 1973 Jan 2;12(1):145–150. doi: 10.1021/bi00725a024. [DOI] [PubMed] [Google Scholar]
- Maxwell I. H., Van Ness J., Hahn W. E. Assay of DNA-RNA hybrids by S1 nuclease digestion and adsorption to DEAE-cellulose filters. Nucleic Acids Res. 1978 Jun;5(6):2033–2038. doi: 10.1093/nar/5.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Davidson E. H., Britten R. J. A program for least squares analysis of reassociation and hybridization data. Nucleic Acids Res. 1977 Jun;4(6):1727–1737. doi: 10.1093/nar/4.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B., Wolk C. P. High recovery of nitrogenase activity and of Fe-labeled nitrogenase in heterocysts isolated from Anabaena variabilis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6271–6275. doi: 10.1073/pnas.75.12.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. D. Macromolecular composition of spores from the filamentous cyanobacterium A nabaena cylindrica. J Bacteriol. 1977 Feb;129(2):1154–1155. doi: 10.1128/jb.129.2.1154-1155.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida-Yasumoto C., Doi R. H. Transcription from the complementary deoxyribonucleic acid strands of Bacillus subtilis during various stages of sporulation. J Bacteriol. 1974 Feb;117(2):775–782. doi: 10.1128/jb.117.2.775-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Perlman R. L., Pastan I. Regulation of lac messenger ribonucleic acid synthesis by cyclic adenosine 3',5'-monophosphate and glucose. J Biol Chem. 1970 May 10;245(9):2259–2267. [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]


