Abstract
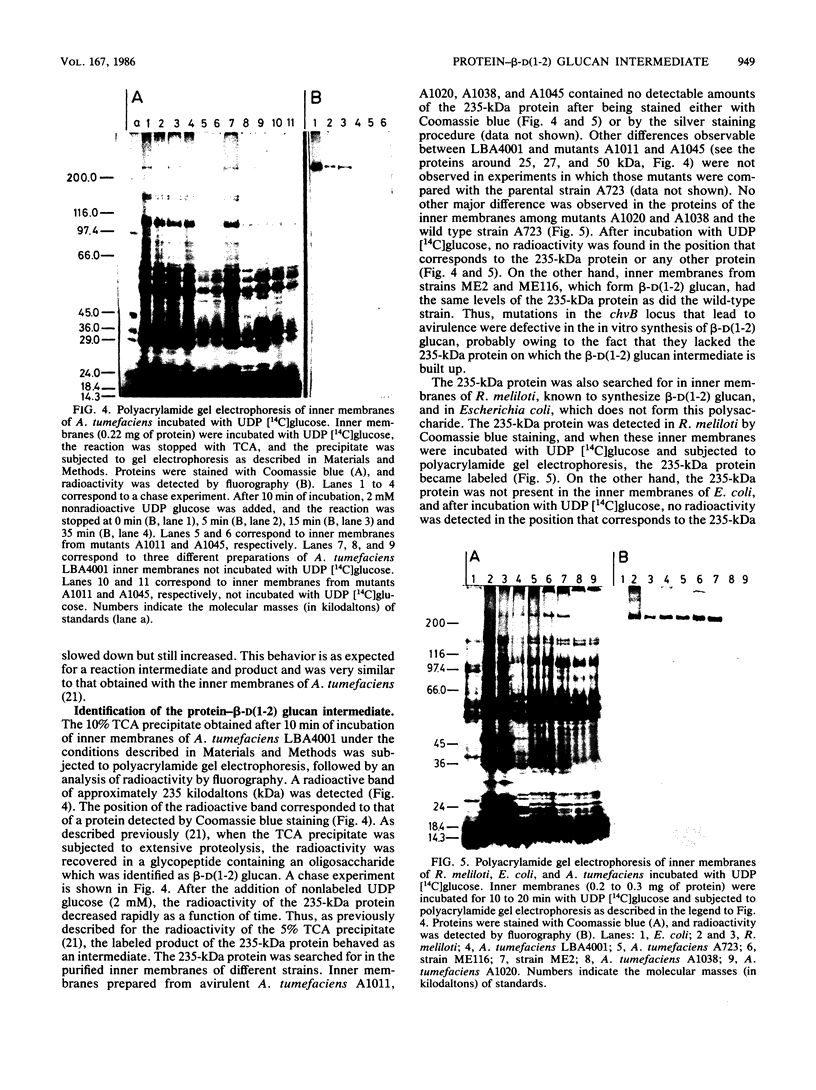
beta-D(1-2) Glucan was synthesized by Agrobacterium and Rhizobium spp. in vitro with enzymes from the internal membranes upon the addition of UDF glucose and Mg2+ or Mn2+. An intermediate containing protein and beta-D(1-2) glucan was formed during the reaction. It could be precipitated with trichloroacetic acid or separated by polyacrylamide gel electrophoresis under denaturing conditions. After detection with Coomassie blue or a radioactive substrate, the intermediate appeared as a 235-kilodalton protein. The radioactivity could be chased with a nonradioactive substrate. All strains that formed beta-D(1-2) glucan in vitro formed the 235-kilodalton protein, whereas avirulent, beta-D(1-2) glucan-negative mutants did not synthesize it. Transposon insertions in the chvB locus of strains ME2 and ME116 did not alter the virulence of the strains. These strains were able to form beta-D(1-2) glucan in vitro and synthesize the 235-kilodalton protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee D., Basu M., Choudhury I., Chatterjee G. C. Cell surface carbohydrates of Agrobacterium tumefaciens involved in adherence during crown gall tumor initiation. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1384–1388. doi: 10.1016/0006-291x(81)91977-x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Douglas C. J., Halperin W., Nester E. W. Agrobacterium tumefaciens mutants affected in attachment to plant cells. J Bacteriol. 1982 Dec;152(3):1265–1275. doi: 10.1128/jb.152.3.1265-1275.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. J., Staneloni R. J., Rubin R. A., Nester E. W. Identification and genetic analysis of an Agrobacterium tumefaciens chromosomal virulence region. J Bacteriol. 1985 Mar;161(3):850–860. doi: 10.1128/jb.161.3.850-860.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Hirsch A. M., Leigh J. A., Johansen E., Kuldau G. A., Deegan S., Walker G. C., Signer E. R. Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell. 1985 Apr;40(4):869–877. doi: 10.1016/0092-8674(85)90346-0. [DOI] [PubMed] [Google Scholar]
- García-Patrone M. Bacitracin increases size of parasporal crystals and spores in Bacillus thuringiensis. Mol Cell Biochem. 1985 Oct;68(2):131–137. doi: 10.1007/BF00219377. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Nester E. W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol. 1980 Nov;144(2):732–743. doi: 10.1128/jb.144.2.732-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapwijk P. M., Scheulderman T., Schilperoort R. A. Coordinated regulation of octopine degradation and conjugative transfer of Ti plasmids in Agrobacterium tumefaciens: evidence for a common regulatory gene and separate operons. J Bacteriol. 1978 Nov;136(2):775–785. doi: 10.1128/jb.136.2.775-785.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse A. G., Holmes K. V., Gurlitz R. H. Elaboration of cellulose fibrils by Agrobacterium tumefaciens during attachment to carrot cells. J Bacteriol. 1981 Jan;145(1):583–595. doi: 10.1128/jb.145.1.583-595.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Stacey G., Douglas C. J., Nester E. W. Role for 2-linked-beta-D-glucan in the virulence of Agrobacterium tumefaciens. J Bacteriol. 1985 Oct;164(1):102–106. doi: 10.1128/jb.164.1.102-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley M. H., Bodwin J. S., Lippincott B. B., Lippincott J. A. Role of Agrobacterium cell envelope lipopolysaccharide in infection site attachment. Infect Immun. 1976 Apr;13(4):1080–1083. doi: 10.1128/iai.13.4.1080-1083.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York W. S., McNeil M., Darvill A. G., Albersheim P. Beta-2-linked glucans secreted by fast-growing species of Rhizobium. J Bacteriol. 1980 Apr;142(1):243–248. doi: 10.1128/jb.142.1.243-248.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorreguieta A., Tolmasky M. E., Staneloni R. J. The enzymatic synthesis of beta 1-2 glucans. Arch Biochem Biophys. 1985 May 1;238(2):368–372. doi: 10.1016/0003-9861(85)90176-6. [DOI] [PubMed] [Google Scholar]
- Zorreguieta A., Ugalde R. A., Leloir L. F. An intermediate in cyclic beta 1-2 glucan biosynthesis. Biochem Biophys Res Commun. 1985 Jan 16;126(1):352–357. doi: 10.1016/0006-291x(85)90613-8. [DOI] [PubMed] [Google Scholar]