Abstract
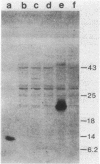
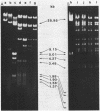
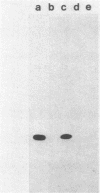
The d gene from the Bacillus subtilis temperate bacteriophage SP beta was isolated. When introduced into an SP beta-sensitive strain of B. subtilis, the cloned d gene directed the synthesis of a 22-kilodalton protein and conferred on the host immunity to SP beta phage. A frameshift mutation, designated d2, was introduced into the cloned d gene, and it was subsequently crossed back into the SP beta phage genome. The resulting SP beta phage grew lytically and formed clear plaques on sensitive bacteria. Although the cloned d gene confers immunity to the host, we could not detect complementation of the d gene by mixed infection with SP beta d2 and various SP beta c mutants. The nucleotide sequence of the 1,033-base-pair PstI-to-EcoRI fragment containing the d gene was determined; it includes an open reading frame that could potentially encode a protein of 227 amino acids. The gene was mapped within the PstI H fragment on the phage genome, which positions the d gene about 25 kilobases from the right end of the phage genome. It is transcribed from right to left.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater K. F., Hopwood D. A., Kieser T., Thompson C. J. Gene cloning in Streptomyces. Curr Top Microbiol Immunol. 1982;96:69–95. doi: 10.1007/978-3-642-68315-2_5. [DOI] [PubMed] [Google Scholar]
- Fink P. S., Korman R. Z., Odebralski J. M., Zahler S. A. Bacillus subtilis bacteriophages SP beta c1 is a deletion mutant of SP beta. Mol Gen Genet. 1981;182(3):514–515. doi: 10.1007/BF00293946. [DOI] [PubMed] [Google Scholar]
- Fink P. S., Zahler S. A. Restriction fragment maps of the genome of Bacillus subtilis bacteriophage SP beta. Gene. 1982 Sep;19(2):235–238. doi: 10.1016/0378-1119(82)90012-9. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Ostroff G. R., Pène J. J. Molecular cloning with bifunctional plasmid vectors in Bacillus subtilis. II. Transfer of sequences propagated in Escherichia coli to B. subtilis. Mol Gen Genet. 1984;193(2):306–311. doi: 10.1007/BF00330685. [DOI] [PubMed] [Google Scholar]
- Palefski S., Hemphill H. E., Kolenbrander P. E., Whiteley H. R. Dominance relationships in mixedly infected Bacillus subtilis. J Virol. 1972 Apr;9(4):594–601. doi: 10.1128/jvi.9.4.594-601.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R., Toye P. A., Korman R. Z., Zahler S. A. The prophage of SP beta c2dcitK1, A defective specialized transducing phage of Bacillus subtilis. Genetics. 1979 Jul;92(3):721–739. doi: 10.1093/genetics/92.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman P., Zuber P., Perkins J. B., Sandman K., Igo M., Losick R. New ways to study developmental genes in spore-forming bacteria. Science. 1985 Apr 19;228(4697):285–291. doi: 10.1126/science.228.4697.285. [DOI] [PubMed] [Google Scholar]
- Zahler S. A., Korman R. Z., Rosenthal R., Hemphill H. E. Bacillus subtilis bacteriophage SPbeta: localization of the prophage attachment site, and specialized transduction. J Bacteriol. 1977 Jan;129(1):556–558. doi: 10.1128/jb.129.1.556-558.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]