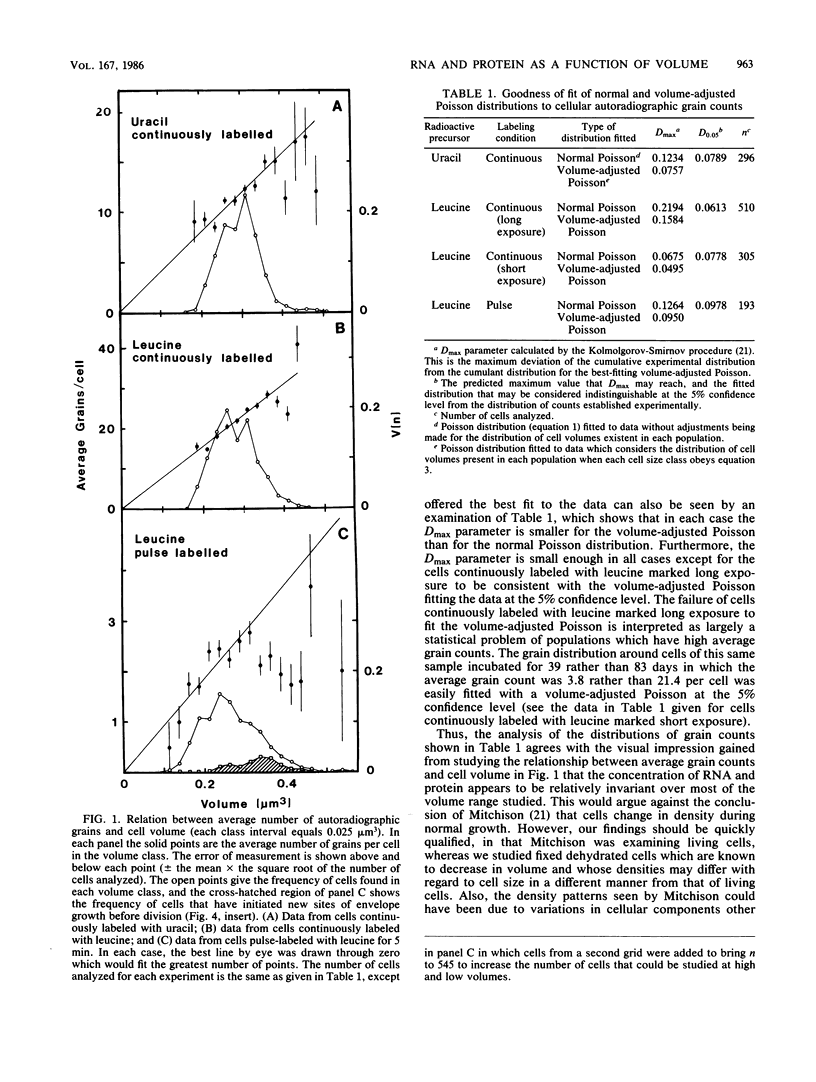
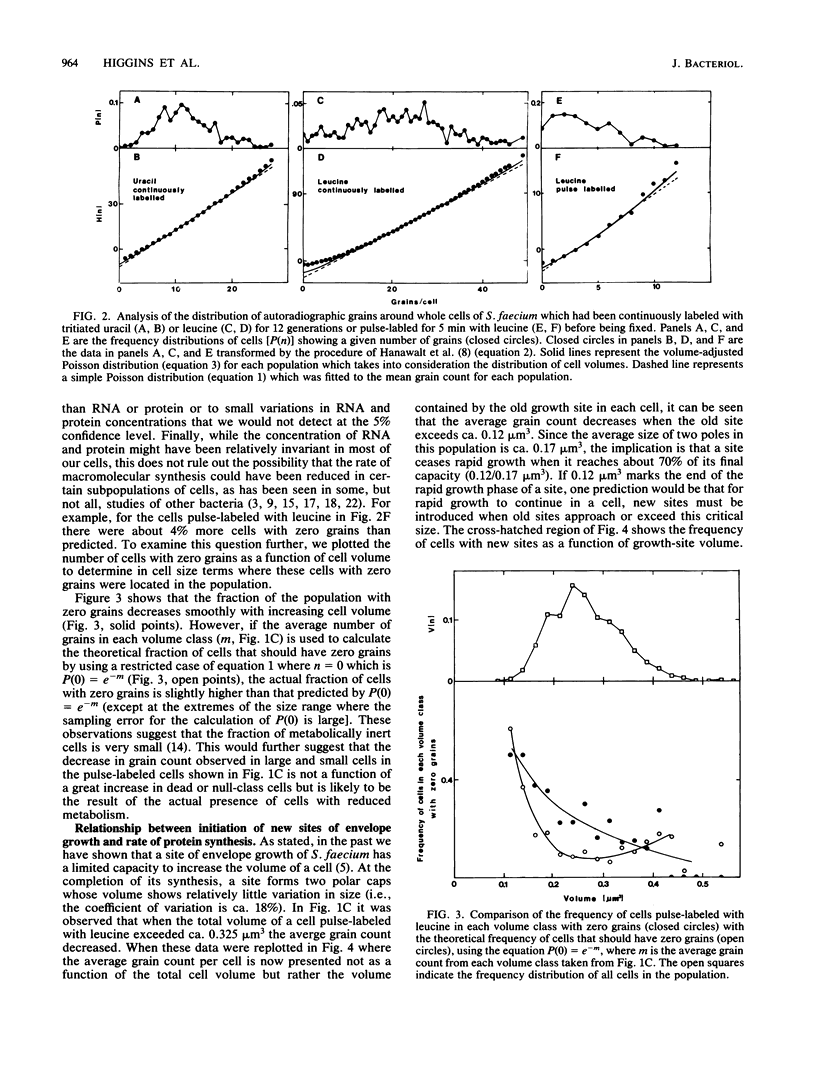
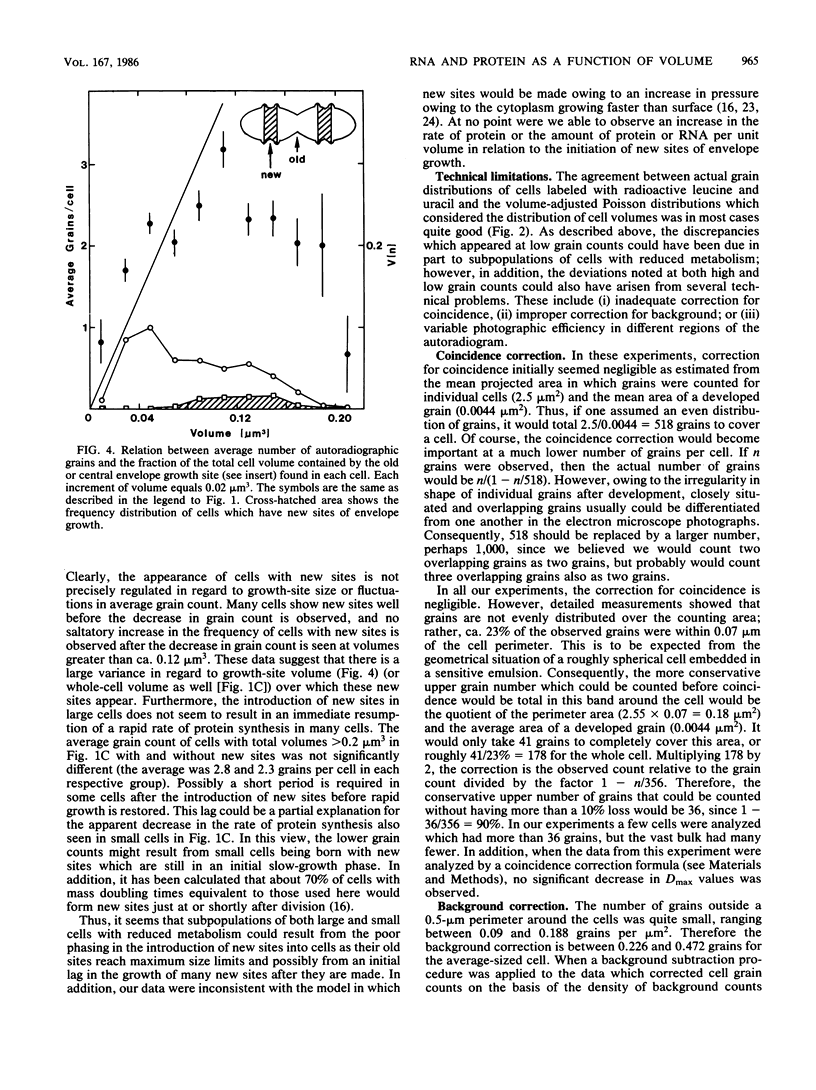
Abstract
Mid-exponential-phase cultures were either labeled continuously with tritiated leucine and uracil or pulse-labeled with tritiated leucine. The amount of leucine and uracil incorporated into protein or RNA per cell was determined by grain counts of autoradiographs of cells seen in electron micrographs; the volume of each cell was determined by three-dimensional reconstruction. The average number of autoradiographic grains around cells continuously labeled with uracil and leucine increased linearly with cell volume. In contrast, while the average grain count around cells pulse-labeled with leucine increased in a near-linear fashion over most of the volume classes, less than the expected number of grains were seen around cells in large- and small-size classes. The distribution of grains around cells from both the continuously and pulse-labeled populations could be fit at the 5% confidence level with a Poisson distribution modified to take into consideration the volume distribution of each population of cells analyzed. These findings suggested that large changes in the density of RNA and protein do not occur in most cells as they increase in size; however, there may be decreases in the rate of protein synthesis in some large and small cells. The decrease in the rate of protein synthesis appears consistent with the hypothesis that new sites of envelope growth must be introduced into cells that are close to the division event to restore rapid growth.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARO L. G., FORRO F., Jr Localization of macromolecules in Escherichia coli. II. RNA and its site of synthesis. J Biophys Biochem Cytol. 1961 Mar;9:555–565. doi: 10.1083/jcb.9.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARO L. G. Localization of macromolecules in Escherichia coli. I. DNA and proteins. J Biophys Biochem Cytol. 1961 Mar;9:539–553. doi: 10.1083/jcb.9.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLINS J. F., RICHMOND M. H. Rate of growth of Bacillus cereus between divisions. J Gen Microbiol. 1962 Apr;28:15–33. doi: 10.1099/00221287-28-1-15. [DOI] [PubMed] [Google Scholar]
- Ecker R. E., Kokaisl G. Synthesis of protein, ribonucleic acid, and ribosomes by individual bacterial cells in balanced growth. J Bacteriol. 1969 Jun;98(3):1219–1226. doi: 10.1128/jb.98.3.1219-1226.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein E. M., Rosenzweig M. S., Daneo-Moore L., Higgins M. L. Unit cell hypothesis for Streptococcus faecalis. J Bacteriol. 1980 Jul;143(1):499–505. doi: 10.1128/jb.143.1.499-505.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson C. W., Daneo-Moore L., Higgins M. L. Initiation of wall assembly sites in Streptococcus faecium. J Bacteriol. 1983 May;154(2):573–579. doi: 10.1128/jb.154.2.573-579.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAWALT P. C., MAALOE O., CUMMINGS D. J., SCHAECHTER M. The normal DNA replication cycle. II. J Mol Biol. 1961 Apr;3:156–165. doi: 10.1016/s0022-2836(61)80042-9. [DOI] [PubMed] [Google Scholar]
- Harvey R. J., Marr A. G., Painter P. R. Kinetics of growth of individual cells of Escherichia coli and Azotobacter agilis. J Bacteriol. 1967 Feb;93(2):605–617. doi: 10.1128/jb.93.2.605-617.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Study of cycle of cell wall assembly in Streptococcus faecalis by three-dimensional reconstructions of thin sections of cells. J Bacteriol. 1976 Sep;127(3):1346–1358. doi: 10.1128/jb.127.3.1346-1358.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L. Three-dimensional reconstruction of whole cells of Streptococcus faecalis from thin sections of cells. J Bacteriol. 1976 Sep;127(3):1337–1345. doi: 10.1128/jb.127.3.1337-1345.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks R. P., Daneo-Moore L., Shockman G. D. Cellular autolytic activity in synchronized populations of Streptococcus faecium. J Bacteriol. 1978 Feb;133(2):822–829. doi: 10.1128/jb.133.2.822-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. Does the initiation of chromosome replication regulate cell division? Adv Microb Physiol. 1977;16:49–98. doi: 10.1016/s0065-2911(08)60047-8. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Does the variability of the cell cycle result from one or many chance events? Nature. 1980 Jul 3;286(5768):80–82. doi: 10.1038/286080a0. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Higgins M. L. Control of wall band splitting in Streptococcus faecalis. J Gen Microbiol. 1984 Apr;130(4):735–745. doi: 10.1099/00221287-130-4-735. [DOI] [PubMed] [Google Scholar]
- Koppes L. H., Woldringh C. L., Nanninga N. Size variations and correlation of different cell cycle events in slow-growing Escherichia coli. J Bacteriol. 1978 May;134(2):423–433. doi: 10.1128/jb.134.2.423-433.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppes L. J., Overbeeke N., Nanninga N. DNA replication pattern and cell wall growth in Escherichia coli PAT 84. J Bacteriol. 1978 Mar;133(3):1053–1061. doi: 10.1128/jb.133.3.1053-1061.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopriwa B. M. A reliable, standardized method for ultrastructural electron microscopic radioautography. Histochemie. 1973 Oct 3;37(1):1–17. doi: 10.1007/BF00306855. [DOI] [PubMed] [Google Scholar]
- MITCHISON J. M. The growth of single cells. III. Streptococcus faecalis. Exp Cell Res. 1961 Jan;22:208–225. doi: 10.1016/0014-4827(61)90099-4. [DOI] [PubMed] [Google Scholar]
- Previc E. P. Biochemical determination of bacterial morphology and the geometry of cell division. J Theor Biol. 1970 Jun;27(3):471–497. doi: 10.1016/s0022-5193(70)80010-8. [DOI] [PubMed] [Google Scholar]
- TOENNIES G., ISZARD L., ROGERS N. B., SHOCKMAN G. D. Cell multiplication studied with an electronic particle counter. J Bacteriol. 1961 Dec;82:857–866. doi: 10.1128/jb.82.6.857-866.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN TUBERGEN R. P., SETLOW R. B. Quantitative radioautographic studies on exponentially growing cultures of Escherichia coli. The distribution of parental DNA, RNA, protein, and cell wall among progeny cells. Biophys J. 1961 Sep;1:589–625. doi: 10.1016/s0006-3495(61)86911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


