Abstract
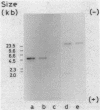
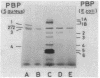
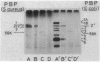
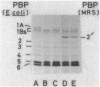
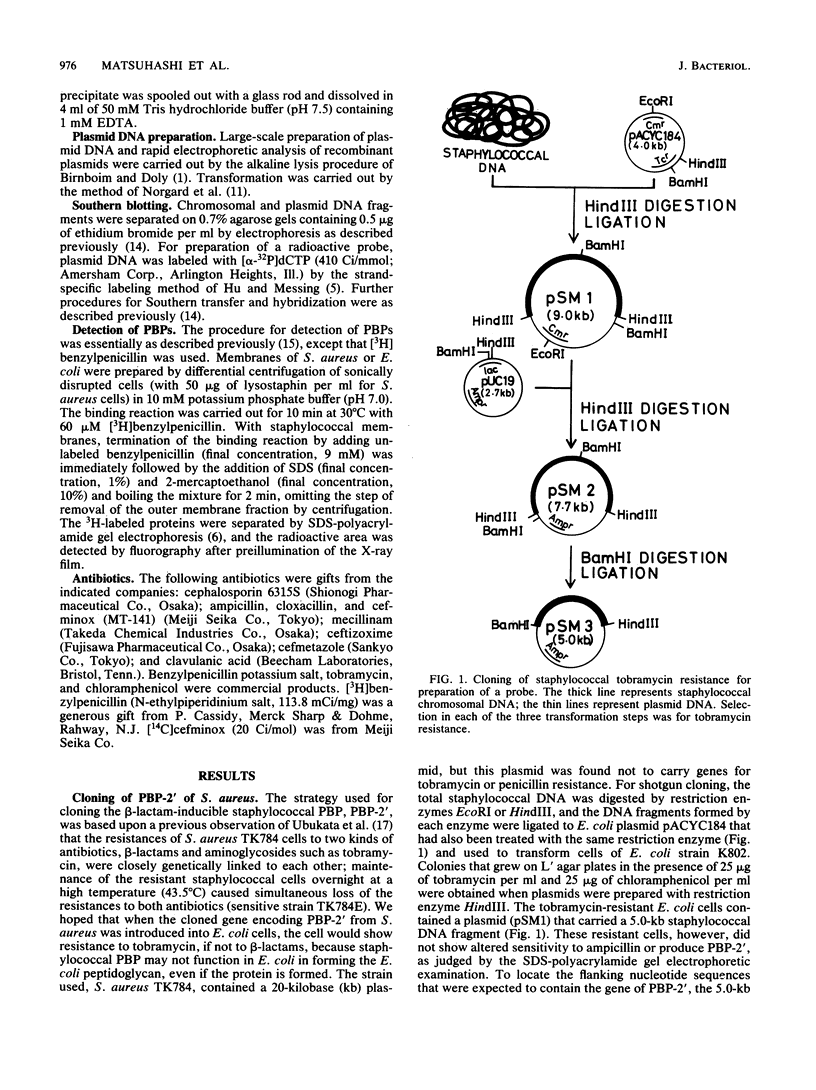
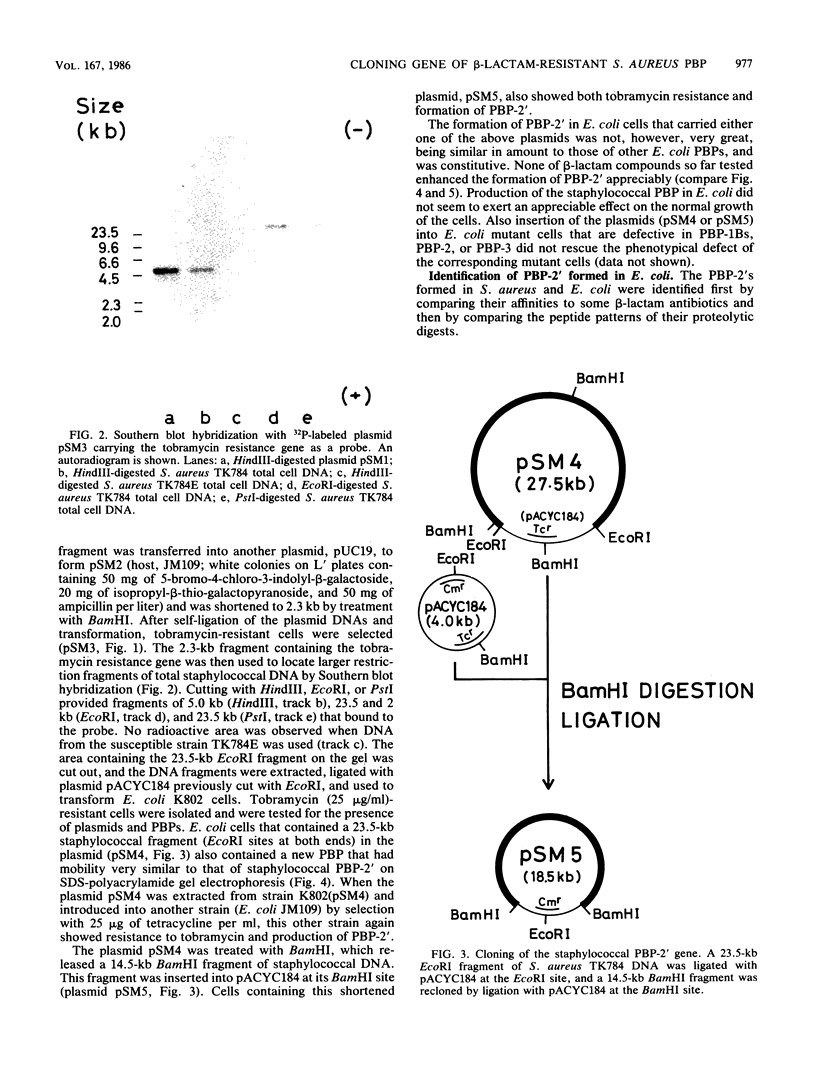
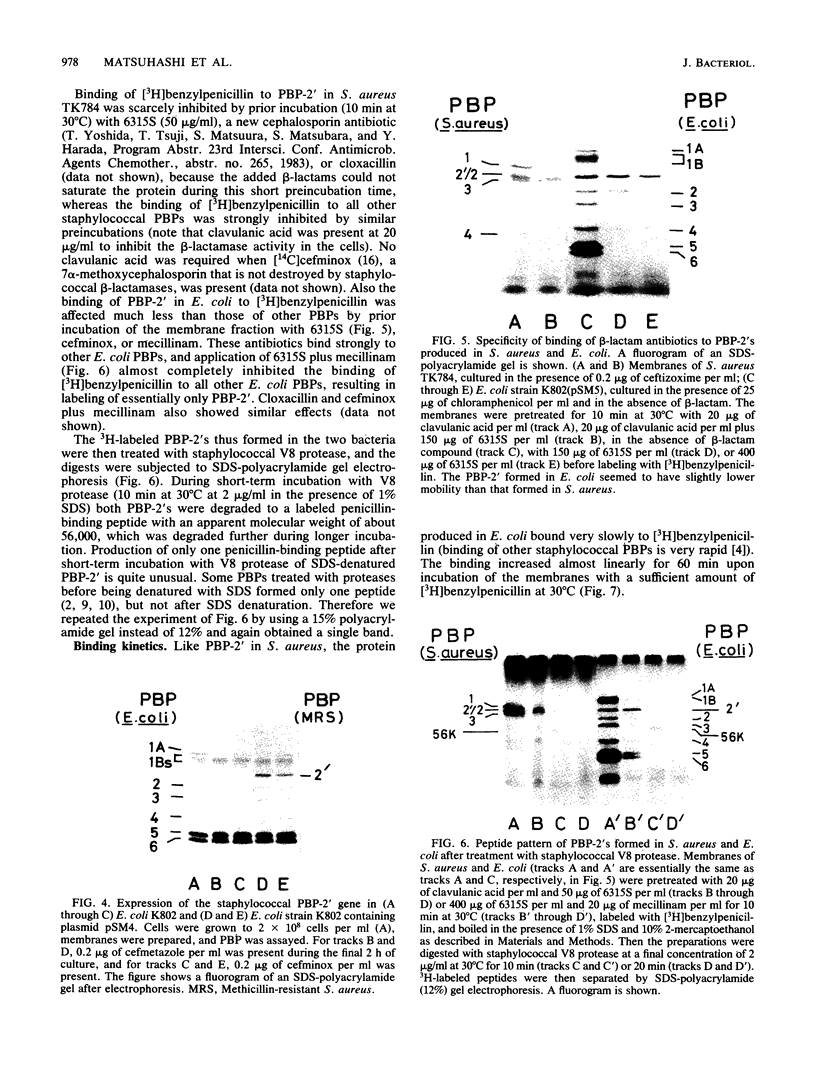
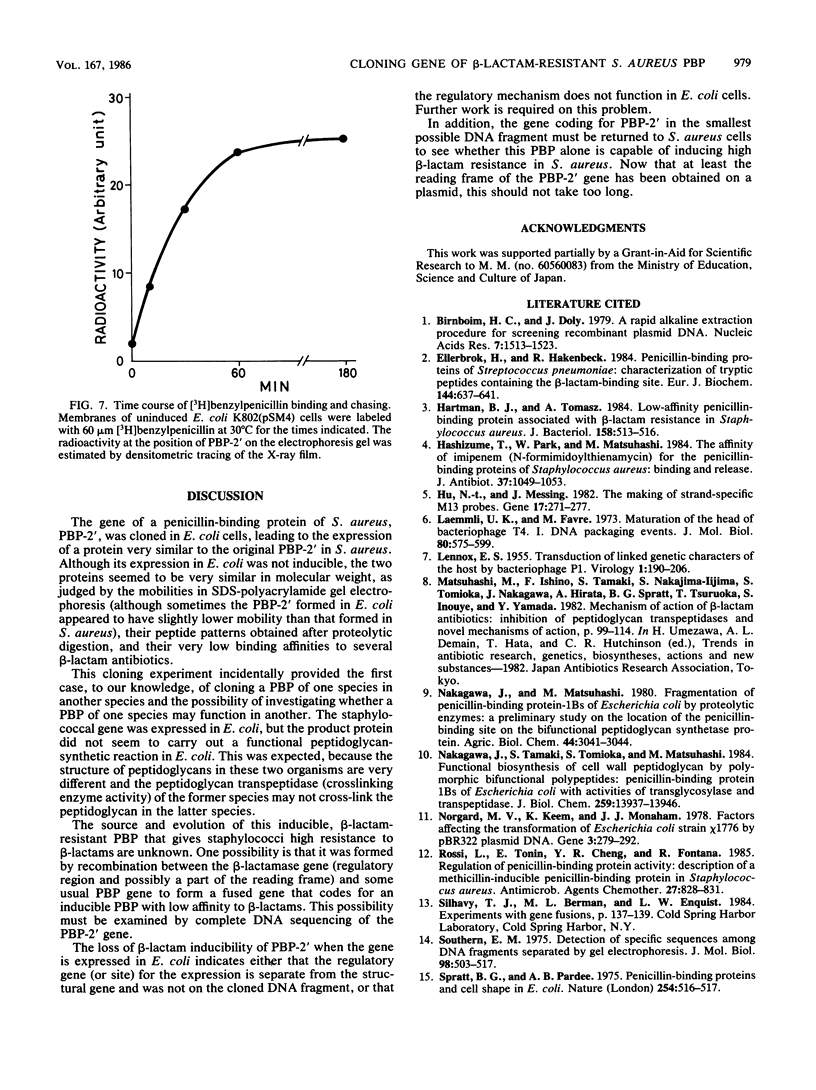
A novel penicillin-binding protein, PBP-2' (Mr about 75,000), is known to be induced in excessively large amount by most beta-lactam compounds in cells of a clinically isolated strain of Staphylococcus aureus, TK784, that is highly resistant to beta-lactams and also most other antibiotics. This protein has very low affinities to most beta-lactam compounds and has been supposed to be the cause of the resistance of the cells to beta-lactams. A 14-kilobase DNA fragment was isolated from the cells that carried the gene encoding this penicillin-binding protein and also a genetically linked marker that is responsible for the resistance to tobramycin. This DNA was cloned on plasmid pACYC184 and was shown to cause both production of PBP-2' and resistance to tobramycin in Escherichia coli cells. However, the formation of PBP-2' in E. coli was only moderate and was independent of normal inducer beta-lactams. The PBP-2' formed in the E. coli cells showed slow kinetics of binding to beta-lactams similar to that of PBP-2' formed in the original S. aureus cells and gave a similar pattern of peptides to the latter when digested with the proteolytic V8 enzyme of S. aureus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerbrok H., Hakenbeck R. Penicillin-binding proteins of Streptococcus pneumoniae: characterization of tryptic peptides containing the beta-lactam-binding site. Eur J Biochem. 1984 Nov 2;144(3):637–641. doi: 10.1111/j.1432-1033.1984.tb08512.x. [DOI] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume T., Park W., Matsuhashi M. The affinity of imipenem (N-formimidoylthienamycin) for the penicillin-binding proteins of Staphylococcus aureus--binding and release. J Antibiot (Tokyo) 1984 Sep;37(9):1049–1053. doi: 10.7164/antibiotics.37.1049. [DOI] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Nakagawa J., Tamaki S., Tomioka S., Matsuhashi M. Functional biosynthesis of cell wall peptidoglycan by polymorphic bifunctional polypeptides. Penicillin-binding protein 1Bs of Escherichia coli with activities of transglycosylase and transpeptidase. J Biol Chem. 1984 Nov 25;259(22):13937–13946. [PubMed] [Google Scholar]
- Norgard M. V., Keem K., Monahan J. J. Factors affecting the transformation of Escherichia coli strain chi1776 by pBR322 plasmid DNA. Gene. 1978 Jul;3(4):279–292. doi: 10.1016/0378-1119(78)90038-0. [DOI] [PubMed] [Google Scholar]
- Rossi L., Tonin E., Cheng Y. R., Fontana R. Regulation of penicillin-binding protein activity: description of a methicillin-inducible penicillin-binding protein in Staphylococcus aureus. Antimicrob Agents Chemother. 1985 May;27(5):828–831. doi: 10.1128/aac.27.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Tsuruoka T., Yamada Y., Goi H., Miyauchi K., Miyata A., Inouye S., Ishino F., Hirata A., Matsuhashi M. The bacteriolytic action of MT-141, a new cephamycin antibiotic, on gram-negative bacteria. J Antimicrob Chemother. 1985 Feb;15(2):159–171. doi: 10.1093/jac/15.2.159. [DOI] [PubMed] [Google Scholar]
- Utsui Y., Yokota T. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985 Sep;28(3):397–403. doi: 10.1128/aac.28.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]