Abstract
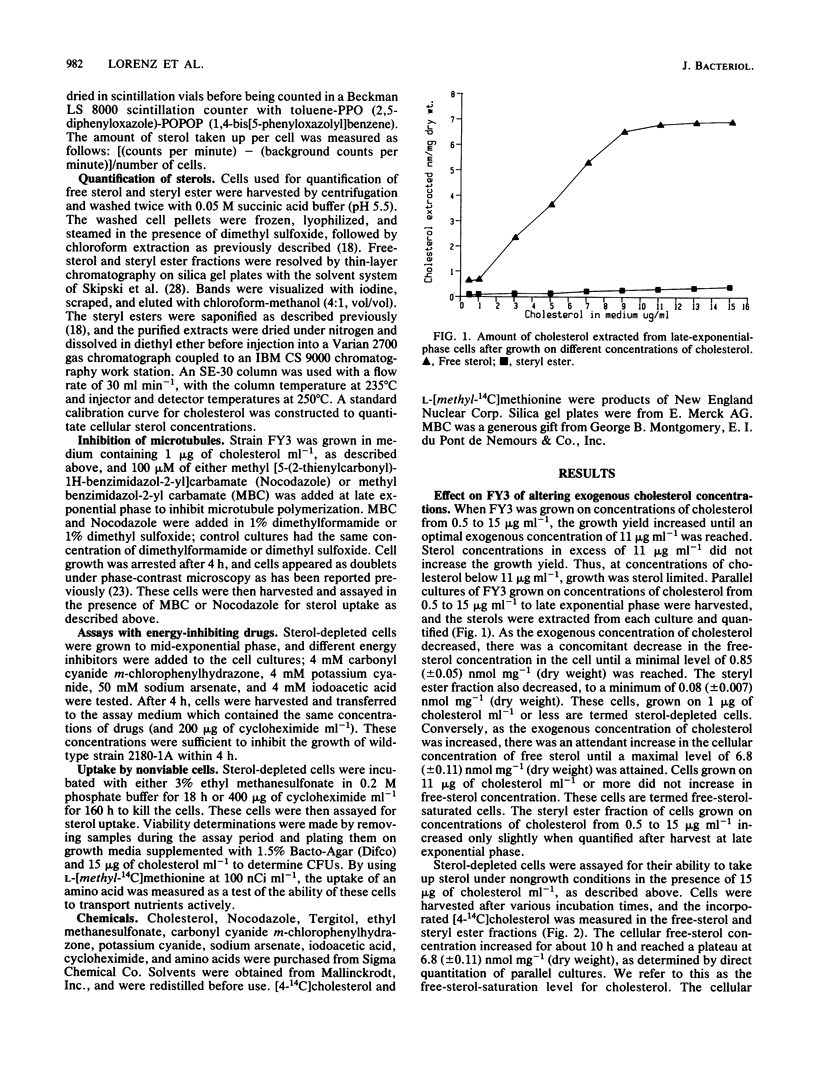
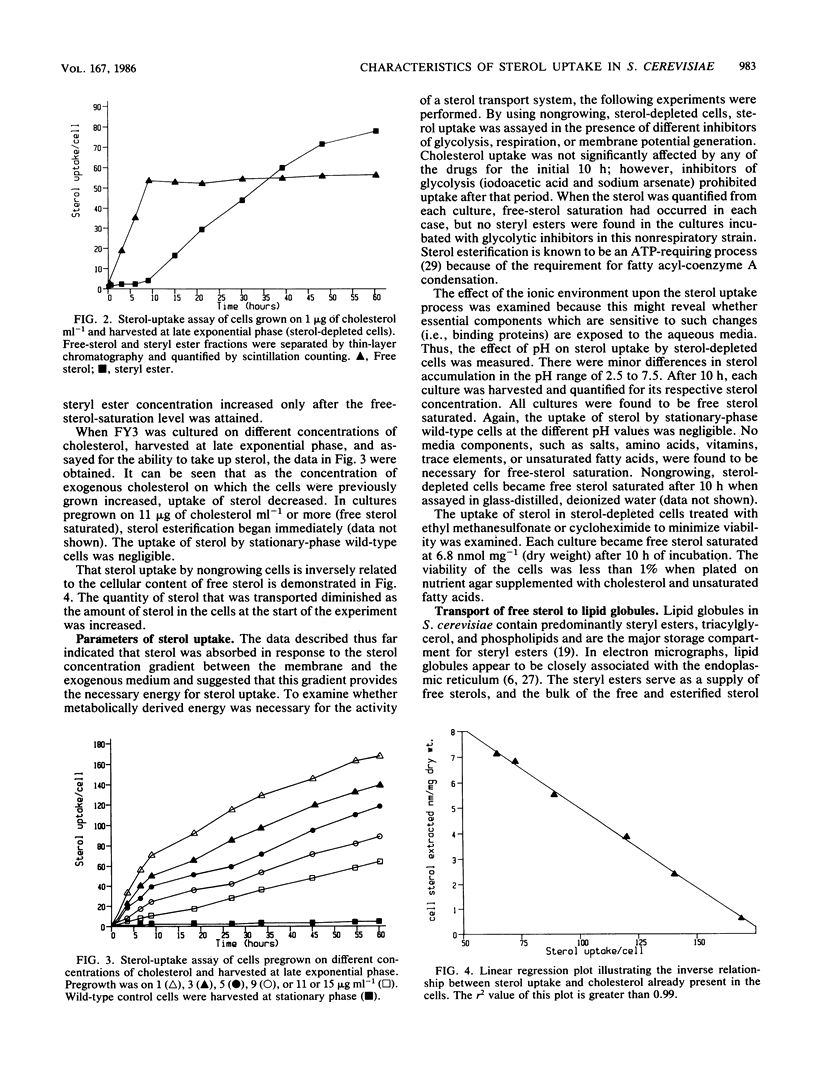
A Saccharomyces cerevisiae sterol auxotroph, FY3 (alpha hem1 erg7 ura), was used to probe the characteristics of sterol uptake in S. cerevisiae. The steady-state cellular concentration of free sterol at the late exponential phase of growth could be adjusted within a 10-fold range by varying the concentration of exogenously supplied sterol. When cultured on 1 microgram of sterol ml-1, the cells contained a minimal cellular free-cholesterol concentration of 0.85 nmol/mg (dry weight) and were termed sterol depleted. When cultured on 11 micrograms of sterol ml-1 or more, the cells contained a maximal cellular free-cholesterol concentration of 6.8 nmol/mg (dry weight) and were termed free sterol saturated. Cells with free-sterol concentrations below the maximal level were capable of accumulating free sterol from the medium. The capacity of the cells for cholesterol uptake was inversely proportional to the initial intracellular concentration. The uptake of sterol was shown to be a nonactive process that is independent of cellular energy sources or viability. The intracellular transport of sterol for esterification is not sensitive to anti-microtubule agents.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREASEN A. A., STIER T. J. B. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J Cell Physiol. 1953 Feb;41(1):23–36. doi: 10.1002/jcp.1030410103. [DOI] [PubMed] [Google Scholar]
- Bottema C. D., Rodriguez R. J., Parks L. W. Influence of sterol structure on yeast plasma membrane properties. Biochim Biophys Acta. 1985 Mar 14;813(2):313–320. doi: 10.1016/0005-2736(85)90247-0. [DOI] [PubMed] [Google Scholar]
- Chambaut-Guérin A. M., Muller P., Rossignol B. Microtubules and protein secretion in rat lacrimal glands. Relationship between colchicine binding and its inhibitory effect on the intracellular transport of proteins. J Biol Chem. 1978 Jun 10;253(11):3870–3876. [PubMed] [Google Scholar]
- Downing J. F., Burrows L. S., Bard M. The isolation of two mutants of Saccharomyces cerevisiae which demonstrate increased activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Biochem Biophys Res Commun. 1980 Jun 16;94(3):974–979. doi: 10.1016/0006-291x(80)91330-3. [DOI] [PubMed] [Google Scholar]
- Gollub E. G., Liu K. P., Dayan J., Adlersberg M., Sprinson D. B. Yeast mutants deficient in heme biosynthesis and a heme mutant additionally blocked in cyclization of 2,3-oxidosqualene. J Biol Chem. 1977 May 10;252(9):2846–2854. [PubMed] [Google Scholar]
- Hata S., Nishino T., Komori M., Katsuki H. Involvement of cytochrome P-450 in delta 22-desaturation in ergosterol biosynthesis of yeast. Biochem Biophys Res Commun. 1981 Nov 16;103(1):272–277. doi: 10.1016/0006-291x(81)91689-2. [DOI] [PubMed] [Google Scholar]
- Lewis T. A., Taylor F. R., Parks L. W. Involvement of heme biosynthesis in control of sterol uptake by Saccharomyces cerevisiae. J Bacteriol. 1985 Jul;163(1):199–207. doi: 10.1128/jb.163.1.199-207.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low C., Rodriguez R. J., Parks L. W. Modulation of yeast plasma membrane composition of a yeast sterol auxotroph as a function of exogenous sterol. Arch Biochem Biophys. 1985 Aug 1;240(2):530–538. doi: 10.1016/0003-9861(85)90059-1. [DOI] [PubMed] [Google Scholar]
- Molzahn S. W., Woods R. A. Polyene resistance and the isolation of sterol mutants in Saccharomyces cerevisiae. J Gen Microbiol. 1972 Sep;72(2):339–348. doi: 10.1099/00221287-72-2-339. [DOI] [PubMed] [Google Scholar]
- Nes W. R., Dhanuka I. C., Pinto W. J. Evidence for facilitated transport in the absorption of sterols by Saccharomyces cerevisiae. Lipids. 1986 Jan;21(1):102–106. doi: 10.1007/BF02534311. [DOI] [PubMed] [Google Scholar]
- Nes W. R., Sekula B. C., Nes W. D., Adler J. H. The functional importance of structural features of ergosterol in yeast. J Biol Chem. 1978 Sep 10;253(17):6218–6225. [PubMed] [Google Scholar]
- Parks L. W., Bottema C. D., Rodriguez R. J., Lewis T. A. Yeast sterols: yeast mutants as tools for the study of sterol metabolism. Methods Enzymol. 1985;111:333–346. doi: 10.1016/s0076-6879(85)11020-7. [DOI] [PubMed] [Google Scholar]
- Parks L. W., McLean-Bowen C., Taylor F. R., Hough S. Sterols in yeast subcellular fractions. Lipids. 1978 Oct;13(10):730–735. doi: 10.1007/BF02533753. [DOI] [PubMed] [Google Scholar]
- Patzelt C., Brown D., Jeanrenaud B. Inhibitory effect of colchicine on amylase secretion by rat parotid glands. Possible localization in the Golgi area. J Cell Biol. 1977 Jun;73(3):578–593. doi: 10.1083/jcb.73.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto W. J., Lozano R., Nes W. R. Inhibition of sterol biosynthesis by ergosterol and cholesterol in Saccharomyces cerevisiae. Biochim Biophys Acta. 1985 Aug 22;836(1):89–95. doi: 10.1016/0005-2760(85)90224-3. [DOI] [PubMed] [Google Scholar]
- Pinto W. J., Nes W. R. Stereochemical specificity for sterols in Saccharomyces cerevisiae. J Biol Chem. 1983 Apr 10;258(7):4472–4476. [PubMed] [Google Scholar]
- Quinlan R. A., Pogson C. I., Gull K. The influence of the microtubule inhibitor, methyl benzimidazol-2-yl-carbamate (MBC) on nuclear division and the cell cycle in Saccharomyces cerevisiae. J Cell Sci. 1980 Dec;46:341–352. doi: 10.1242/jcs.46.1.341. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. J., Low C., Bottema C. D., Parks L. W. Multiple functions for sterols in Saccharomyces cerevisiae. Biochim Biophys Acta. 1985 Dec 4;837(3):336–343. doi: 10.1016/0005-2760(85)90057-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. J., Parks L. W. Structural and physiological features of sterols necessary to satisfy bulk membrane and sparking requirements in yeast sterol auxotrophs. Arch Biochem Biophys. 1983 Sep;225(2):861–871. doi: 10.1016/0003-9861(83)90099-1. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Smolowe A. F., Sullivan R. C., Barclay M. Separation of lipid classes by thin-layer chromatography. Biochim Biophys Acta. 1965 Oct 4;106(2):386–396. doi: 10.1016/0005-2760(65)90047-0. [DOI] [PubMed] [Google Scholar]
- Taketani S., Nishino T., Katsuki H. Characterization of sterol-ester synthetase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1979 Oct 26;575(1):148–155. [PubMed] [Google Scholar]
- Taylor F. R., Parks L. W. Adaptation of Saccharomyces cerevisiae to growth on cholesterol: selection of mutants defective in the formation of lanosterol. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1437–1445. doi: 10.1016/s0006-291x(80)80058-1. [DOI] [PubMed] [Google Scholar]
- Taylor F. R., Parks L. W. An assessment of the specificity of sterol uptake and esterification in Saccharomyces cerevisiae. J Biol Chem. 1981 Dec 25;256(24):13048–13054. [PubMed] [Google Scholar]
- Taylor F. R., Parks L. W. Metabolic interconversion of free sterols and steryl esters in Saccharomyces cerevisiae. J Bacteriol. 1978 Nov;136(2):531–537. doi: 10.1128/jb.136.2.531-537.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trocha P. J., Sprinson D. B. Location and regulation of early enzymes of sterol biosynthesis in yeast. Arch Biochem Biophys. 1976 May;174(1):45–51. doi: 10.1016/0003-9861(76)90322-2. [DOI] [PubMed] [Google Scholar]



