Abstract
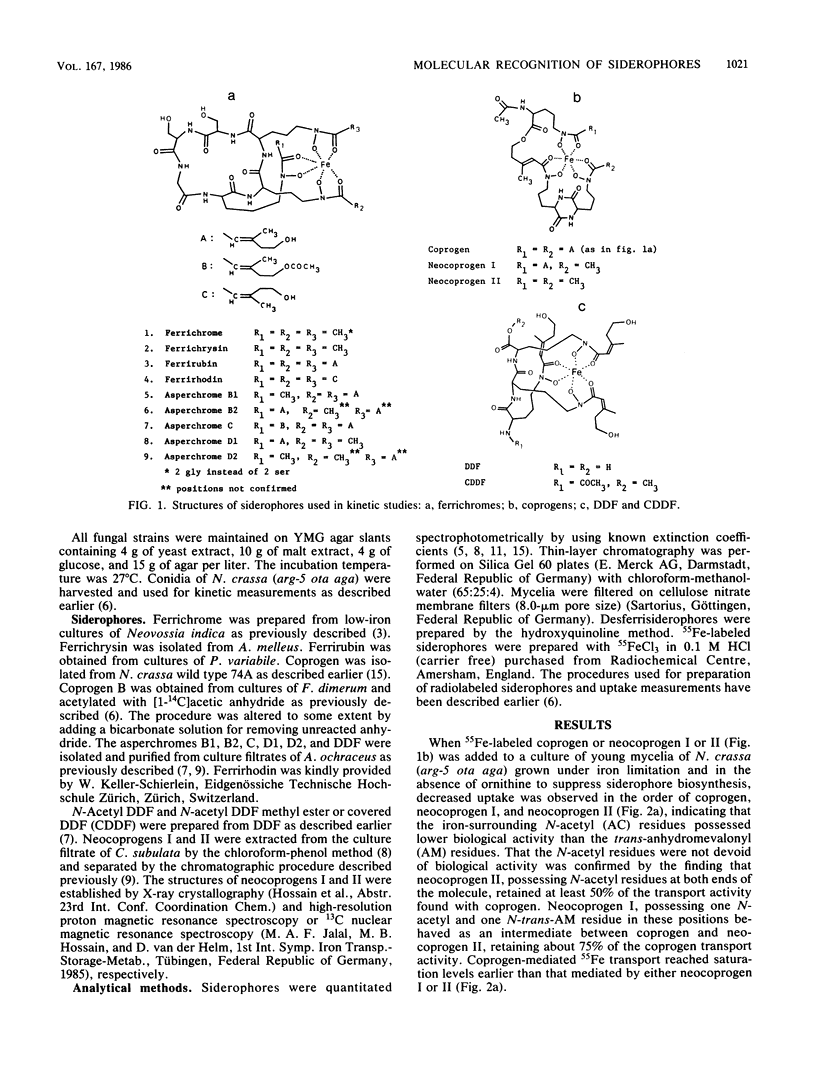
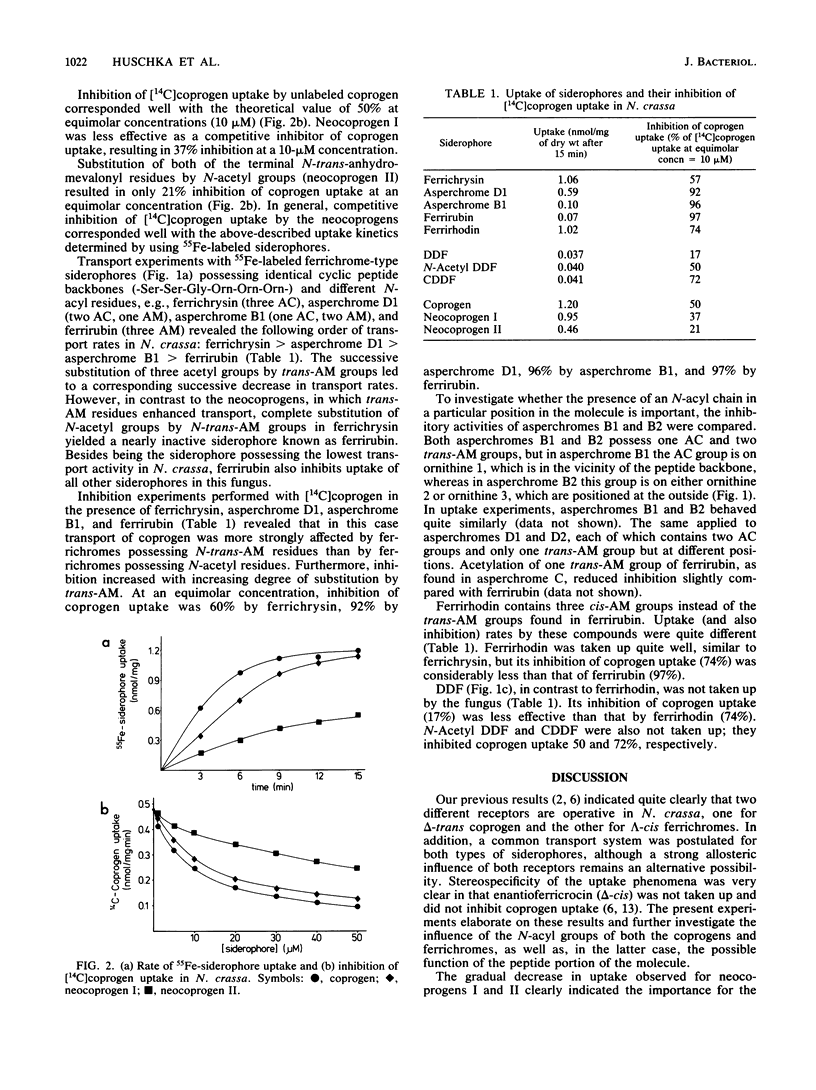
Recognition of ferric siderophores in Neurospora crassa was found to depend on the number and kind of N-acyl residues that surrounded the iron coordination center. In the coprogen series, uptake decreased in the order of coprogen, neocoprogen I, and neocoprogen II, indicating that gradual replacement of the N-transanhydromevalonyl groups by N-acetyl groups had an adverse effect on uptake. The reverse effect was observed in the ferrichrome series, where uptake decreased in the order of ferrichrysin, asperchrome D1, asperchrome B1, and ferrirubin. Configuration of the anhydromevalonyl group (cis or trans) in ferrichromes was also an important determinant in the recognition process. On the basis of uptake and inhibition studies, it is proposed that in ferrichromes part of the molecule (iron configuration and the N-acyl groups) is responsible for binding, whereas another (cyclic peptide ring) is involved in the subsequent process of transport.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chung T. D., Matzanke B. F., Winkelmann G., Raymond K. N. Inhibitory effect of the partially resolved coordination isomers of chromic desferricoprogen on coprogen uptake in Neurospora crassa. J Bacteriol. 1986 Jan;165(1):283–287. doi: 10.1128/jb.165.1.283-287.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery T., Emery L., Olsen R. K. Retrohydroxamate ferrichrome, a biomimetic analogue of ferrichrome. Biochem Biophys Res Commun. 1984 Mar 30;119(3):1191–1197. doi: 10.1016/0006-291x(84)90902-1. [DOI] [PubMed] [Google Scholar]
- Huschka H., Naegeli H. U., Leuenberger-Ryf H., Keller-Schierlein W., Winkelmann G. Evidence for a common siderophore transport system but different siderophore receptors in Neurospora crassa. J Bacteriol. 1985 May;162(2):715–721. doi: 10.1128/jb.162.2.715-721.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal M. A., Mocharla R., Barnes C. L., Hossain M. B., Powell D. R., Eng-Wilmot D. L., Grayson S. L., Benson B. A., van der Helm D. Extracellular siderophores from Aspergillus ochraceous. J Bacteriol. 1984 May;158(2):683–688. doi: 10.1128/jb.158.2.683-688.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal M. A., Mocharla R., van der Helm D. Separation of ferrichromes and other hydroxamate siderophores of fungal origin by reversed-phase chromatography. J Chromatogr. 1984 Sep 28;301(1):247–252. doi: 10.1016/s0021-9673(01)89192-5. [DOI] [PubMed] [Google Scholar]
- Zalkin A., Forrester J. D., Templeton D. H. Ferrichrome-A tetrahydrate. Determination of crystal and molecular structure. J Am Chem Soc. 1966 Apr 20;88(8):1810–1814. doi: 10.1021/ja00960a040. [DOI] [PubMed] [Google Scholar]



