Abstract
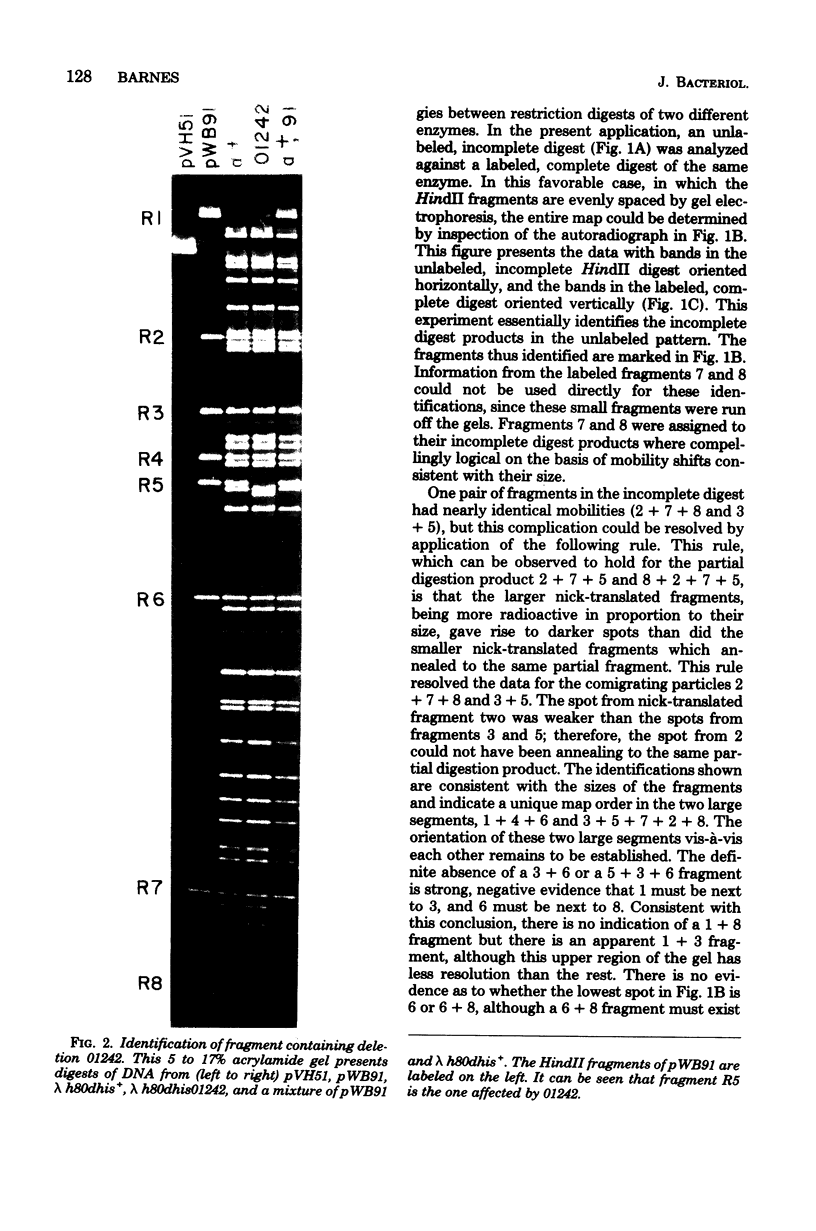
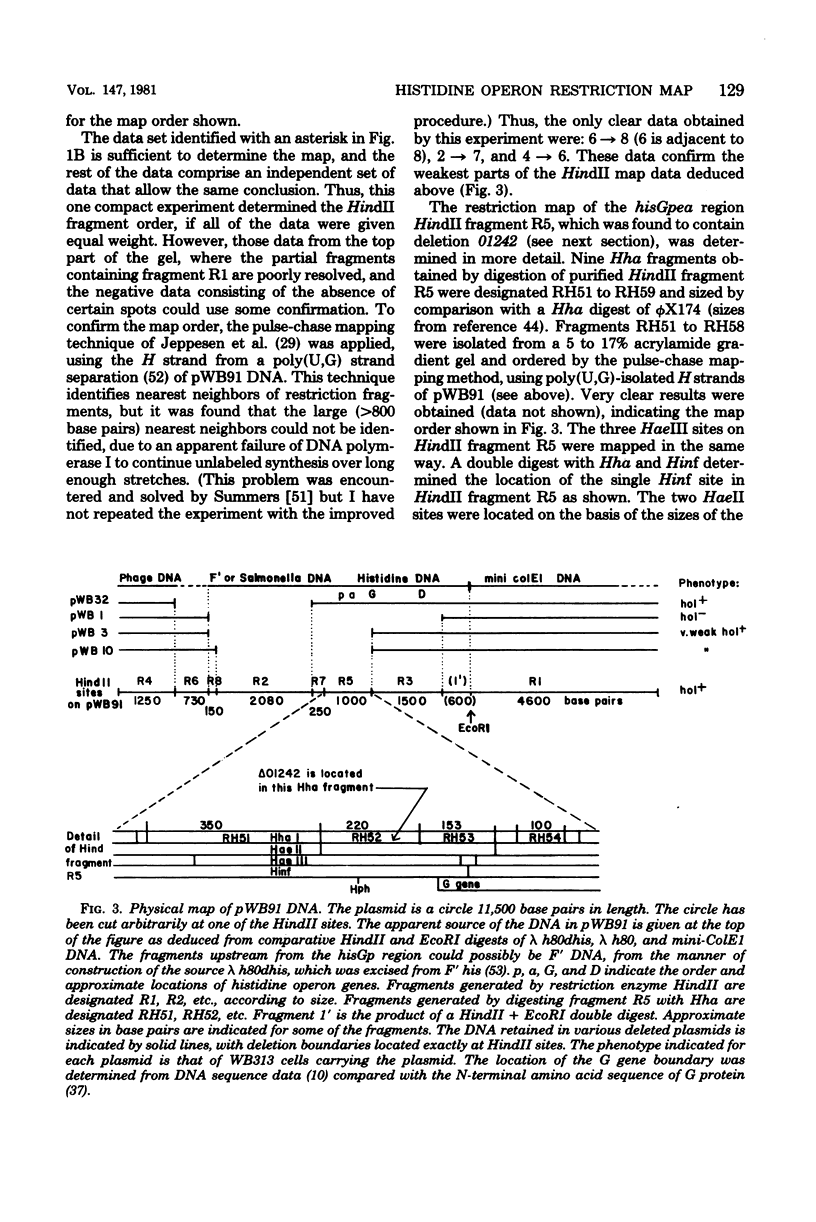
The first part of the histidine operon of Salmonella typhimurium, hisGpeaGD, has been cloned onto the vector plasmid mini-ColE1 (pVH51). The resulting plasmid, pWB91, has a single EcoRI site and is 11,500 base pairs in size. The HindII restriction map was determined by the method of two-dimensional cross-annealing between a partial digest pattern and a complete digest pattern. The restriction fragment containing the genetic control region was identified with the aid of the small (35-base pair) internal deletion 01242 and the observation that heteroduplexed restriction fragments containing this deletion have markedly reduced mobility on polyacrylamide gels. The genetic control region was then mapped in more detail with other restriction enzymes. The genetic orientation of the restriction map was determined with the aid of several deletions of integral HindII fragments generated in vitro.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M., Sanger F., Coulson A. R. Nucleotide and amino acid sequences of gene G of omegaX174. J Mol Biol. 1976 Dec 15;108(3):519–533. doi: 10.1016/s0022-2836(76)80134-9. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Anderson R. P., Roth J. R. Tandem chromosomal duplications in Salmonella typhimurium: fusion of histidine genes to novel promoters. J Mol Biol. 1978 Feb 15;119(1):147–166. doi: 10.1016/0022-2836(78)90274-7. [DOI] [PubMed] [Google Scholar]
- Anderson R. P., Roth J. R. Tandem genetic duplications in Salmonella typhimurium: amplification of the histidine operon. J Mol Biol. 1978 Nov 25;126(1):53–71. doi: 10.1016/0022-2836(78)90279-6. [DOI] [PubMed] [Google Scholar]
- Artz S. W., Broach J. R. Histidine regulation in Salmonella typhimurium: an activator attenuator model of gene regulation. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3453–3457. doi: 10.1073/pnas.72.9.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M. Construction of an M13 histidine-transducing phage: a single-stranded cloning vehicle with one EcoRI site. Gene. 1979 Feb;5(2):127–139. doi: 10.1016/0378-1119(79)90098-2. [DOI] [PubMed] [Google Scholar]
- Barnes W. M. DNA sequence from the histidine operon control region: seven histidine codons in a row. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4281–4285. doi: 10.1073/pnas.75.9.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M. DNA sequencing by partial ribosubstitution. J Mol Biol. 1978 Feb 15;119(1):83–99. doi: 10.1016/0022-2836(78)90271-1. [DOI] [PubMed] [Google Scholar]
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Barnes W. N., Reznikoff W. S. The isolation of RNA homologous to the genetic control elements of the lactose operon. J Biol Chem. 1975 Oct 25;250(20):8184–8192. [PubMed] [Google Scholar]
- Brownlee G. G., Cartwright E. M. Rapid gel sequencing of RNA by primed synthesis with reverse transcriptase. J Mol Biol. 1977 Jul;114(1):93–117. doi: 10.1016/0022-2836(77)90285-6. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B., Fankhauser D. B., Hartman P. E. A fine structure map of the salmonella histidine operator-promoter. Genetics. 1974 Oct;78(2):607–631. doi: 10.1093/genetics/78.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B. Physiological studies of salmonella histidine operator-promoter mutants. Genetics. 1974 Oct;78(2):593–606. doi: 10.1093/genetics/78.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S. G., Lerman L. S. Length-independent separation of DNA restriction fragments in two-dimensional gel electrophoresis. Cell. 1979 Jan;16(1):191–200. doi: 10.1016/0092-8674(79)90200-9. [DOI] [PubMed] [Google Scholar]
- Fischer S. G., Lerman L. S. Separation of random fragments of DNA according to properties of their sequences. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4420–4424. doi: 10.1073/pnas.77.8.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield L., Simpson L., Kaplan D. Conversion of closed circular DNA molecules to single-nicked molecules by digestion with DNAase I in the presence of ethidium bromide. Biochim Biophys Acta. 1975 Oct 15;407(3):365–375. doi: 10.1016/0005-2787(75)90104-5. [DOI] [PubMed] [Google Scholar]
- HARTMAN P. E., LOPER J. C., SERMAN D. Fine structure mapping by complete transduction between histidine-requiring Salmonella mutants. J Gen Microbiol. 1960 Apr;22:323–353. doi: 10.1099/00221287-22-2-323. [DOI] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Chow L., Helinski D. R. Characterization of a mini-ColC1 plasmid. J Bacteriol. 1976 Apr;126(1):447–453. doi: 10.1128/jb.126.1.447-453.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe I., Johnston H. M., Biek D., Roth J. R. A refined map of the hisG gene of Salmonella typhimurium. Genetics. 1979 May;92(1):17–26. doi: 10.1093/genetics/92.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B., Dawson J. H., Desiderio R., Mosher C. W. Ethidium analogues with improved resolution in the dye-buoyant density procedure. Nucleic Acids Res. 1977;4(5):1349–1359. doi: 10.1093/nar/4.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. G., Sanders L., Slocombe P. M. A restriction cleavage map of phiX 174 DNA by pulse-chase labelling using E. coli DNA polymerase. Nucleic Acids Res. 1976 May;3(5):1323–1329. doi: 10.1093/nar/3.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. G. Separation and isolation of DNA fragments using linear polyacrylamide gradient gel electrophoresis. Methods Enzymol. 1980;65(1):305–319. doi: 10.1016/s0076-6879(80)65041-1. [DOI] [PubMed] [Google Scholar]
- Johnston H. M., Barnes W. M., Chumley F. G., Bossi L., Roth J. R. Model for regulation of the histidine operon of Salmonella. Proc Natl Acad Sci U S A. 1980 Jan;77(1):508–512. doi: 10.1073/pnas.77.1.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston H. M., Roth J. R. DNA sequence changes of mutations altering attenuation control of the histidine operon of Salmonella typhimurium. J Mol Biol. 1981 Feb 5;145(4):735–756. doi: 10.1016/0022-2836(81)90312-0. [DOI] [PubMed] [Google Scholar]
- Kasai T. Regulation of the expression of the histidine operon in Salmonella typhimurium. Nature. 1974 Jun 7;249(457):523–527. doi: 10.1038/249523a0. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenow H., Overgaard-Hansen K., Patkar S. A. Proteolytic cleavage fo native DNA polymerase into two different catalytic fragments. Influence of assay condtions on the change of exonuclease activity and polymerase activity accompanying cleavage. Eur J Biochem. 1971 Oct 14;22(3):371–381. doi: 10.1111/j.1432-1033.1971.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Ames B. N. Histidine regulation in Salmonella typhimurium. XI. The percentage of transfer RNA His charged in vivo and its relation to the repression of the histidine operon. J Mol Biol. 1972 Apr 28;66(1):131–142. doi: 10.1016/s0022-2836(72)80011-1. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piszkiewicz D., Tilley B. E., Rand-Meir T., Parsons S. M. Amino acid sequence of ATP phosphoribosyltransferase of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1589–1592. doi: 10.1073/pnas.76.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON C. C., SCHILDKRAUT C. L., APOSHIAN H. V., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XIV. FURTHER PURIFICATION AND PROPERTIES OF DEOXYRIBONUCLEIC ACID POLYMERASE OF ESCHERICHIA COLI. J Biol Chem. 1964 Jan;239:222–232. [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff W. S., Winter R. B., Hurley C. K. The location of the repressor binding sites in the lac operon. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2314–2318. doi: 10.1073/pnas.71.6.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J., Breitmeyer J. B., Tabachnik N. F., Myers P. A. A second specific endonuclease from Haemophilus aegyptius. J Mol Biol. 1975 Jan 5;91(1):121–123. doi: 10.1016/0022-2836(75)90375-7. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction endonucleases. CRC Crit Rev Biochem. 1976 Nov;4(2):123–164. doi: 10.3109/10409237609105456. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Antón D. N., Hartman P. E. Histidine regulatory mutants in Salmonella typhimurium. I. Isolation and general properties. J Mol Biol. 1966 Dec 28;22(2):305–323. doi: 10.1016/0022-2836(66)90134-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Setlow P., Brutlag D., Kornberg A. Deoxyribonucleic acid polymerase: two distinct enzymes in one polypeptide. I. A proteolytic fragment containing the polymerase and 3' leads to 5' exonuclease functions. J Biol Chem. 1972 Jan 10;247(1):224–231. [PubMed] [Google Scholar]
- Sgaramella V. Enzymatic oligomerization of bacteriophage P22 DNA and of linear Simian virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3389–3393. doi: 10.1073/pnas.69.11.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lund E., Smithies O., Blattner F. R. Hybridization of labeled RNA to DNA in agarose gels. Nucleic Acids Res. 1975 Oct;2(10):1911–1929. doi: 10.1093/nar/2.10.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R., Tong B. Construction of phi80 dhis carrying Salmonella typhimurium histidine operon mutations. J Bacteriol. 1974 Dec;120(3):1223–1226. doi: 10.1128/jb.120.3.1223-1226.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sugino A., Goodman H. M., Heyneker H. L., Shine J., Boyer H. W., Cozzarelli N. R. Interaction of bacteriophage T4 RNA and DNA ligases in joining of duplex DNA at base-paired ends. J Biol Chem. 1977 Jun 10;252(11):3987–3994. [PubMed] [Google Scholar]
- Voll M. J. Derivation of an F-merogenote and a phi-80 high-frequency transducing phage carrying the histidine operon os Salmonella. J Bacteriol. 1972 Feb;109(2):741–750. doi: 10.1128/jb.109.2.741-750.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox G., Singer J., Heffernan L. Deoxyribonucleic acid-ribonucleic acid hybridization studies on the L-Arabinose operon of Escherichia coli B-r. J Bacteriol. 1971 Oct;108(1):1–4. doi: 10.1128/jb.108.1.1-4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]








