Abstract
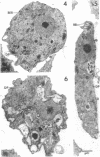
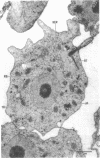
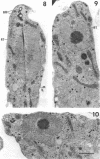
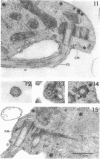
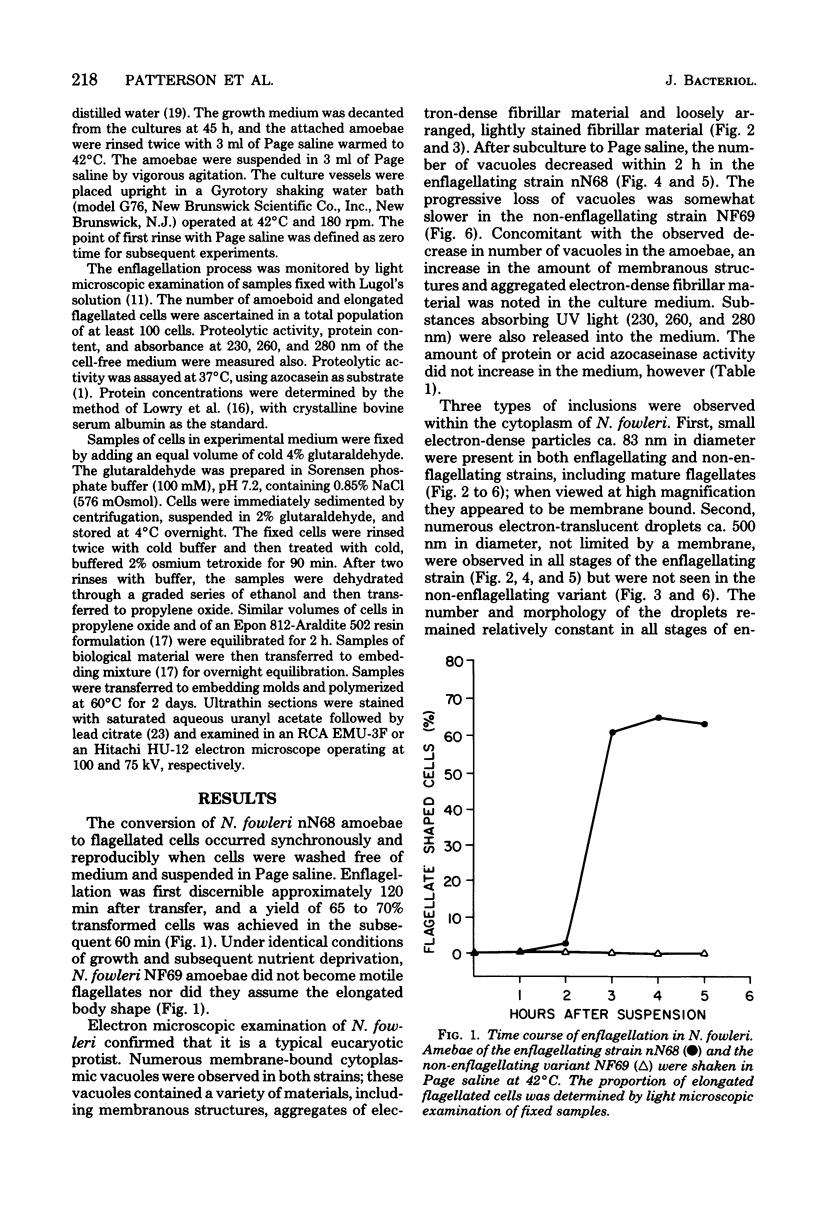
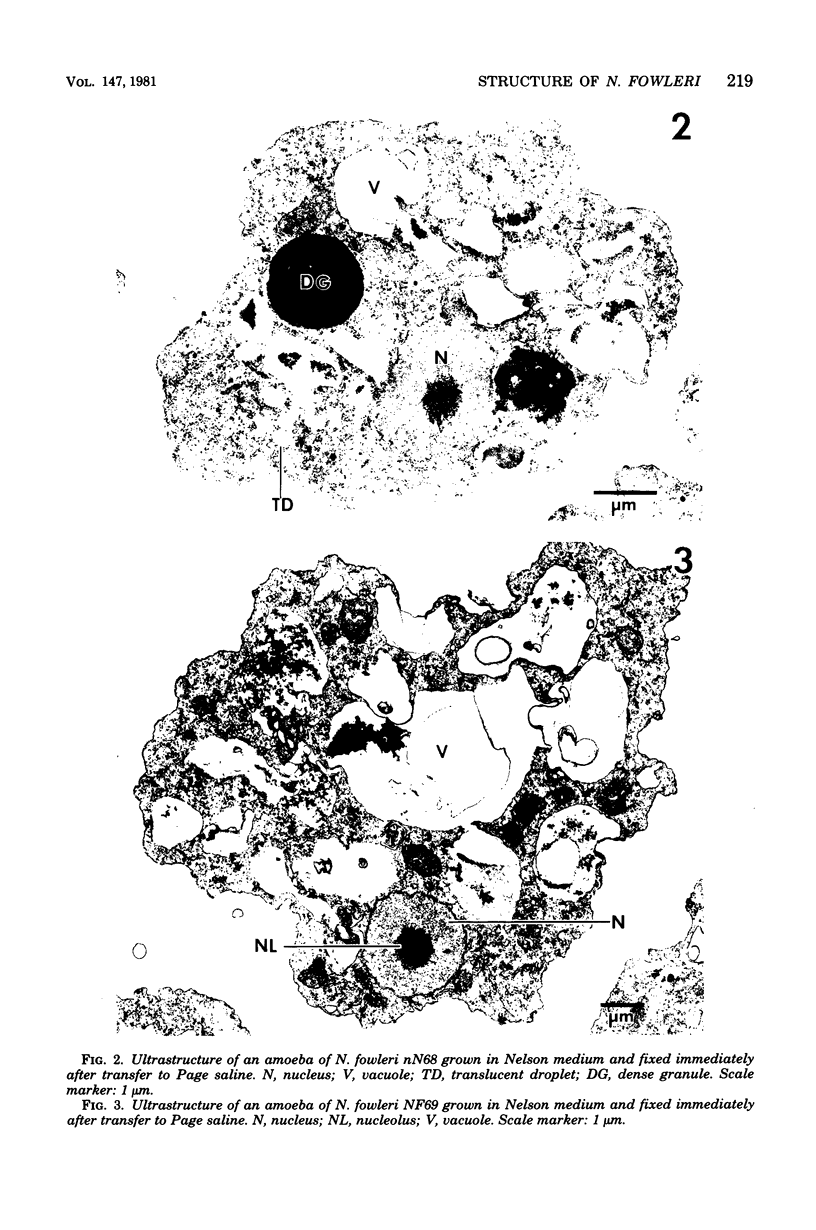
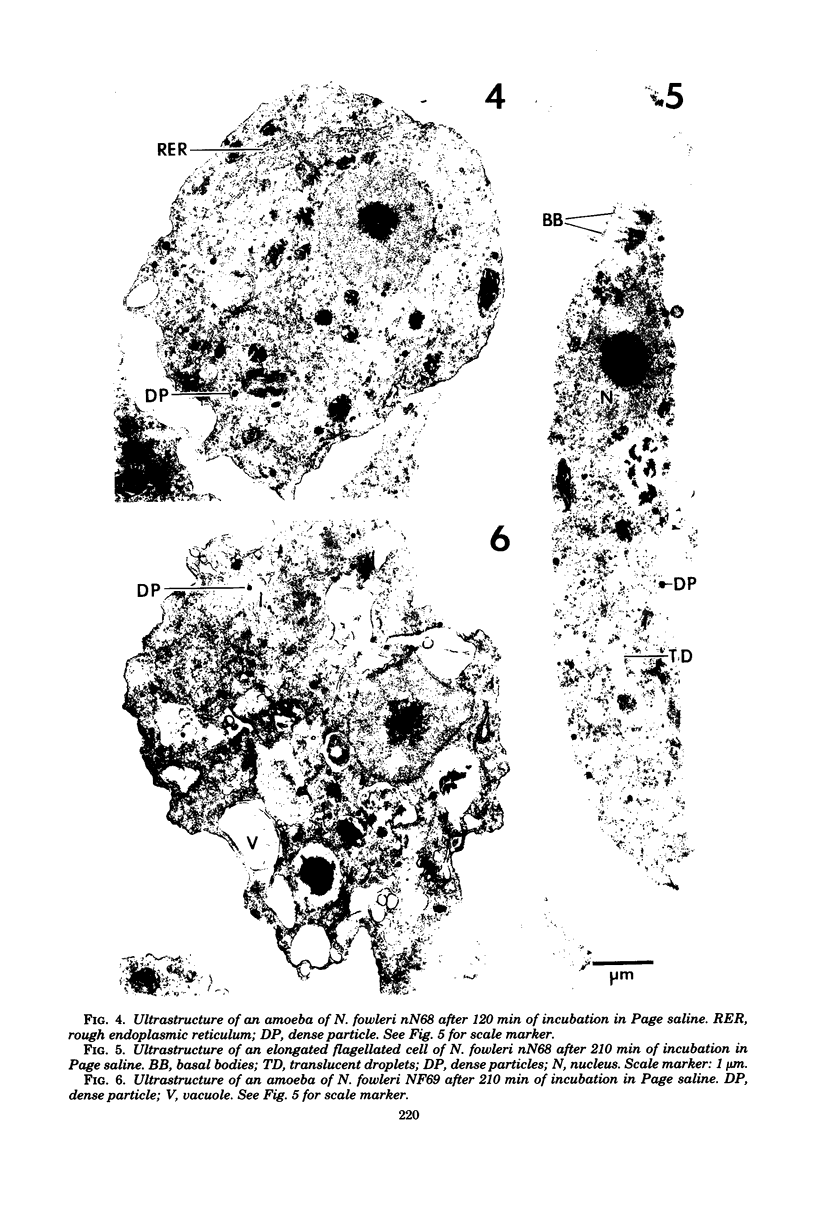
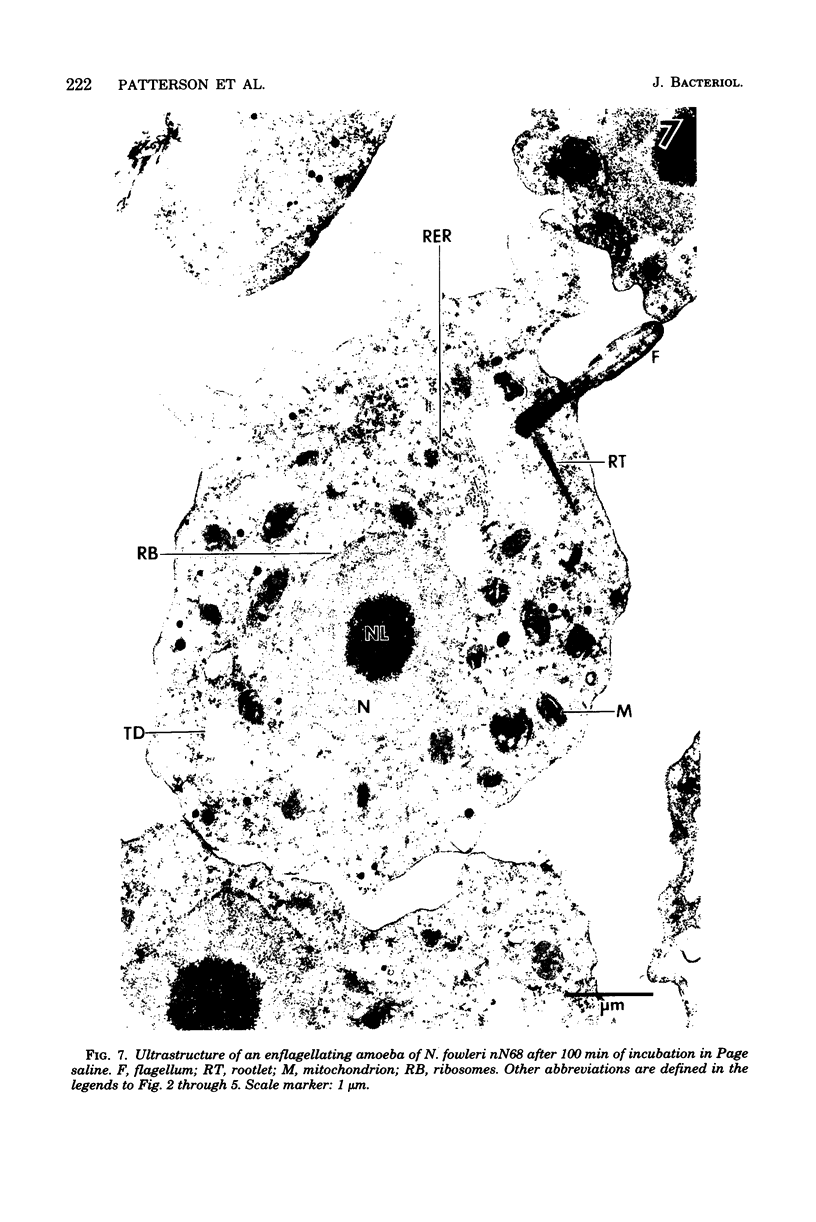
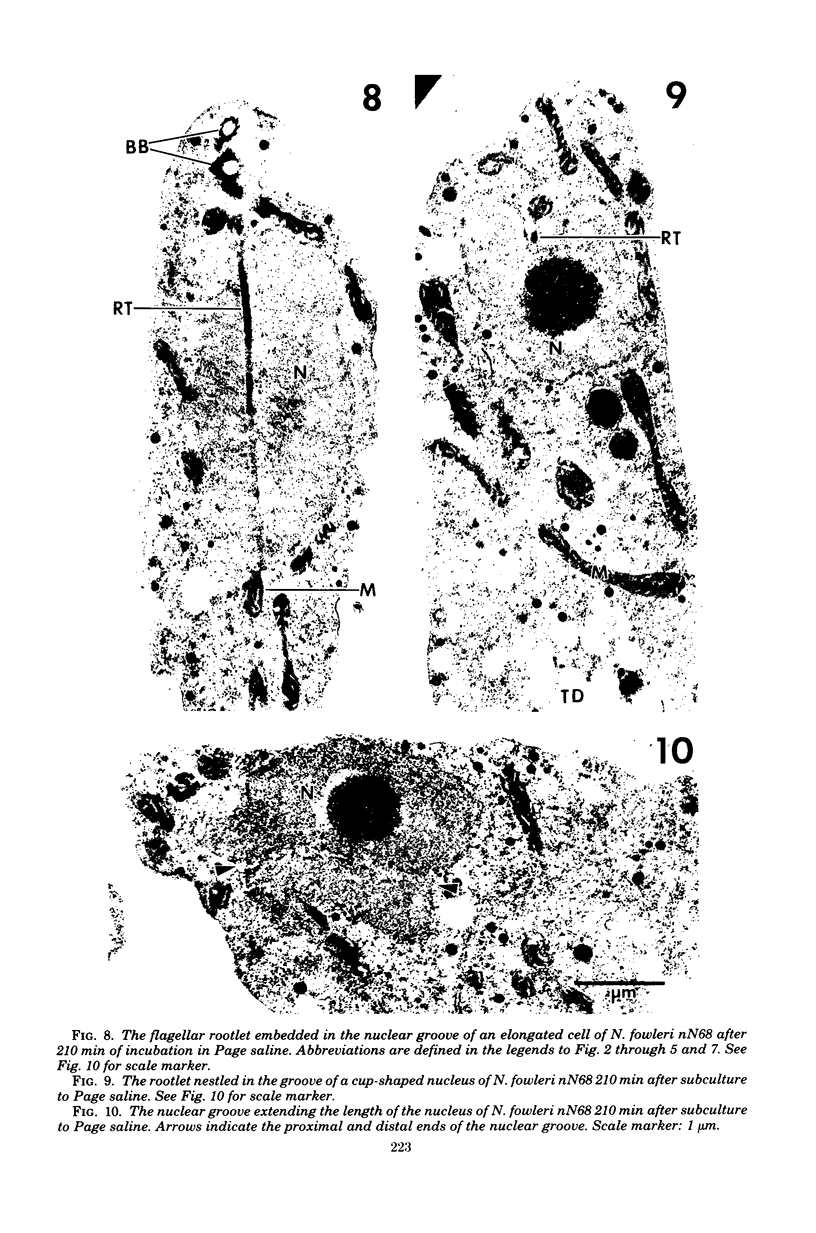
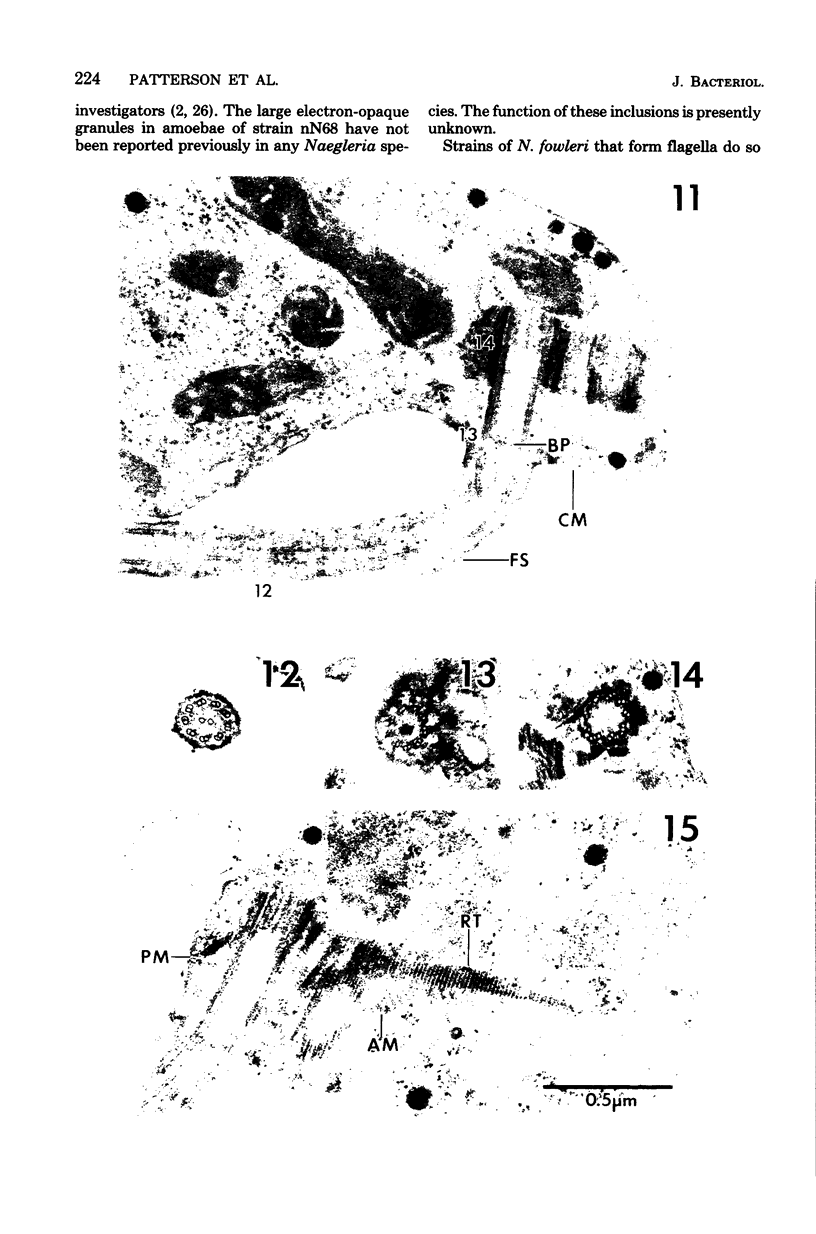
Amoebae of Naegleria fowleri nN68 became elongated flagellated cells 150 to 180 min after subculture to non-nutrient buffer. N. fowleri NF69 did not become elongated or flagellated under these conditions. Electron microscopic examination of N. fowleri confirmed that it is a typical eucaryotic protist with a distinct nuclear envelope and prominent nucleolus, numerous vacuoles and cytoplasmic inclusions, pleomorphic mitochondria, and some rough endoplasmic reticulum. During incubation in non-nutrient buffer, both strains lost ultraviolet-absorbing material to the medium, and the number of vacuoles decreased. In strain nN68, basal bodies, a rootlet, and flagella are formed quickly after an initial lag of 90 min. Initially, the rootlet is not associated with the nucleus but they become associated subsequent at the leading end of the elongated cell. In elongated cells, the rootlet lies in a furrow or groove extending the length of the nucleus. Flagella of N. fowleri nN68 exhibit the typical 9 + 2 arrangement of filaments and are surrounded by a sheath which is continuous with the plasma membrane. The enflagellation process in N. fowleri can be manipulated reproducibly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beynon R. J., Kay J. The inactivation of native enzymes by a neutral proteinase from rat intestinal muscle. Biochem J. 1978 Jul 1;173(1):291–298. doi: 10.1042/bj1730291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R. F. Description of a Naegleria sp. isolated from two cases of primary amoebic meningo-encephalitis, and of the experimental pathological changes induced by it. J Pathol. 1970 Apr;100(4):217–244. doi: 10.1002/path.1711000402. [DOI] [PubMed] [Google Scholar]
- Carter R. F. Primary amoebic meningo-encephalitis. An appraisal of present knowledge. Trans R Soc Trop Med Hyg. 1972;66(2):193–213. doi: 10.1016/0035-9203(72)90147-2. [DOI] [PubMed] [Google Scholar]
- Chang S. L. Small, free-living amebas: cultivation, quantitation, identification, classification, pathogenesis, and resistance. Curr Top Comp Pathobiol. 1971;1:201–254. doi: 10.1016/b978-0-12-153401-1.50010-7. [DOI] [PubMed] [Google Scholar]
- Culbertson C. G. The pathogenicity of soil amebas. Annu Rev Microbiol. 1971;25:231–254. doi: 10.1146/annurev.mi.25.100171.001311. [DOI] [PubMed] [Google Scholar]
- Dingle A. D., Fulton C. Development of the flagellar apparatus of Naegleria. J Cell Biol. 1966 Oct;31(1):43–54. doi: 10.1083/jcb.31.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duma R. J., Rosenblum W. I., McGehee R. F., Jones M. M., Nelson E. C. Primary amoebic meningoencephalitis caused by Naegleria. Two new cases, response to amphotericin B, and a review. Ann Intern Med. 1971 Jun;74(6):923–931. doi: 10.7326/0003-4819-74-6-923. [DOI] [PubMed] [Google Scholar]
- Fulton C. Cell differentiation in Naegleria gruberi. Annu Rev Microbiol. 1977;31:597–629. doi: 10.1146/annurev.mi.31.100177.003121. [DOI] [PubMed] [Google Scholar]
- Fulton C., Dingle A. D. Appearance of the flagellate phenotype in populations of Naegleria amebae. Dev Biol. 1967 Feb;15(2):165–191. doi: 10.1016/0012-1606(67)90012-7. [DOI] [PubMed] [Google Scholar]
- Fulton C. Intracellular regulation of cell shape and motility in Naegleria. First insights and a working hypothesis. J Supramol Struct. 1977;6(1):13–43. doi: 10.1002/jss.400060103. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Pacheco G., Tulloch G. S. Microfilariae of Dirofilaria striata in a dog. J Parasitol. 1970 Apr;56(2):248–248. [PubMed] [Google Scholar]
- Page F. C. Taxonomic criteria for limax amoebae, with descriptions of 3 new species of Hartmannella and 3 of Vahlkampfia. J Protozool. 1967 Aug;14(3):499–521. doi: 10.1111/j.1550-7408.1967.tb02036.x. [DOI] [PubMed] [Google Scholar]
- Pringle H. L., Bradley S. G., Harris L. S. Susceptibility of Naegleria fowleri to delta 9-tetrahydrocannabinol. Antimicrob Agents Chemother. 1979 Nov;16(5):674–679. doi: 10.1128/aac.16.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster F. L., Dunnebacke T. H. Growth at 37 degrees C of the EGs strain of the amoeboflagellate Naegleria gruberi containing viruslike particles. II. Cytoplasmic changes. J Invertebr Pathol. 1974 Mar;23(2):182–189. doi: 10.1016/0022-2011(74)90182-7. [DOI] [PubMed] [Google Scholar]
- Stevens A. R., De Jonckheere J., Willaert E. Naegleria lovaniensis new species: isolation and identification of six thermophilic strains of a new species found in association with Naegleria fowleri. Int J Parasitol. 1980 Feb;10(1):51–64. doi: 10.1016/0020-7519(80)90064-8. [DOI] [PubMed] [Google Scholar]
- Visvesvara G. S., Callaway C. S. Light and electron microsopic observations on the pathogenesis of Naegleria fowleri in mouse brain and tissue culture. J Protozool. 1974 May;21(2):239–250. doi: 10.1111/j.1550-7408.1974.tb03648.x. [DOI] [PubMed] [Google Scholar]
- Weik R. R., John D. T. Agitated mass cultivation of Naegleria fowleri. J Parasitol. 1977 Oct;63(5):868–871. [PubMed] [Google Scholar]
- Weik R. R., John D. T. Quantitation and cell size of Naegleria fowleri by electronic particle counting. J Parasitol. 1977 Feb;63(1):150–151. [PubMed] [Google Scholar]