Abstract
Oxidation of endogenous macromolecules can generate electrophiles capable of forming mutagenic adducts in DNA. The lipid peroxidation product malondialdehyde, for example, reacts with DNA to form M1G, the mutagenic pyrimidopurinone adduct of deoxyguanosine. In addition to free radical attack of lipids, DNA is also continuously subjected to oxidative damage. Among the products of oxidative DNA damage are base propenals. We hypothesized that these structural analogs of malondialdehyde would react with DNA to form M1G. Consistent with this hypothesis, we detected a dose-dependent increase in M1G in DNA treated with calicheamicin and bleomycin, oxidizing agents known to produce base propenal. The hypothesis was proven when we determined that 9-(3-oxoprop-1-enyl)adenine gives rise to the M1G adduct with greater efficiency than malondialdehyde itself. The reactivity of base propenals to form M1G and their presence in the target DNA suggest that base propenals derived from oxidative DNA damage may contribute to the mutagenic burden of a cell.
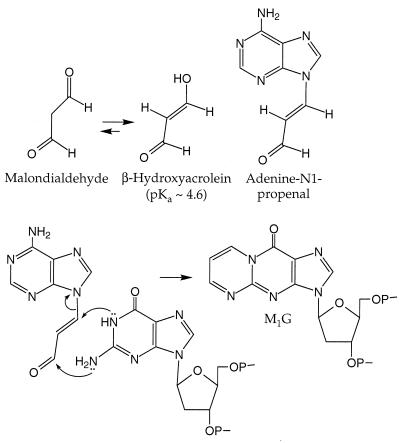
Oxidation of biological molecules has been implicated in both cancer and aging (1, 2). The genotoxicity of oxidizing agents, both exogenous and endogenous, arises from direct damage to DNA (3, 4) or from reactions with other biomolecules that lead to the formation of DNA-reactive electrophilic species (5–7). The lipid peroxidation product malondialdehyde is an example of the indirect pathway (8). Malondialdehyde reacts with DNA to form a variety of adducts, the most abundant of which is M1G (Fig. 1), the pyrimidopurinone of deoxyguanosine (5, 9). The M1G adduct is mutagenic (10–12) and has been detected at levels of ≈5000 adducts/cell in normal human liver (5). We now report that M1G adducts can also be formed in a reaction of DNA with base propenals derived from oxidative DNA damage.
Figure 1.
Structures of malondialdehyde, its tautomer, adenine propenal, and M1G and a proposed mechanism for the formation of M1G from adenine propenal.
Lipids represent an important target for free radical attack (13). However, it is estimated that DNA in a human cell is subjected to thousands of oxidative insults per day from oxygen radicals and related species that arise from normal metabolism and inflammation (2). Even with efficient DNA repair, oxidation of deoxyribose and possibly the bases results in the formation of several electrophilic and potentially genotoxic products (3, 4). For example, base propenal (Fig. 1) is one of the products arising from abstraction of the deoxyribose 4′-hydrogen atom by hydroxyl radical (3) and other activated oxygen species (14), as well as several DNA-cleaving antibiotics (15). Base propenals have been shown to be lethal to cells at micromolar concentrations (16, 17), but the genotoxicity of these and other products of oxidative deoxyribose damage have been largely unexplored.
The genotoxic potential of a base propenal was predicted from its structural identity as a β-substituted acrolein, which is analogous to the enolate form of malondialdehyde (Fig. 1). Previous studies demonstrated that certain β-substituted acroleins are significantly more mutagenic than malondialdehyde (12). To test the hypothesis that base propenals would react with DNA to form M1G, we have examined the formation of this adduct in DNA treated with adenine propenal and with two oxidizing agents known to produce base propenal, bleomycin and calicheamicin (15). Our results suggest that, in addition to the direct genotoxicity of oxidative DNA lesions, the base propenal product of deoxyribose oxidation may also contribute to the mutagenic burden of a cell.
MATERIALS AND METHODS
Reagents.
9-(3-Oxoprop-1-enyl)adenine (adenine-N1-propenal, adenine propenal) was purchased from Salford Ultrafine Chemicals (Manchester, U.K.). Blenoxane (a mixture of bleomycins A2 and B2) and calicheamicin γ1I were generously provided by Terry Doyle (Bristol-Myers Squibb) and George Ellestad (Wyeth Ayerst Research, Pearl River, NY), respectively. Calf thymus DNA (Sigma) was prepared by rehydration in water, shearing with a 20-gauge needle, purification with a Qiagen Gigaprep kit, and dialysis against 50 mM sodium phosphate (pH 7). All other chemicals were reagent grade or better.
Reactions of DNA with Oxidizing Agents and Adenine Propenal.
The calicheamicin reaction was performed by adding a methanolic solution of the drug (5 or 20 μM final concentrations) to calf thymus DNA (50 μg/ml or 950 μg/ml) in 50 mM sodium phosphate (pH 7), with 10 mM glutathione present to activate the drug. Reaction of bleomycin with DNA was performed by premixing an aqueous solution of blenoxane with ferrous ammonium sulfate at a molar ratio of 4:5 and adding the mixture to a solution of DNA in 50 mM sodium phosphate (pH 7). In all cases, the reactions occurred at ambient temperature for 1 hr followed by overnight dialysis at 4°C against 50 mM Hepes/1 mM EDTA (pH 7). DNA was treated with adenine propenal by adding an aliquot of the base propenal in dimethyl sulfoxide to a 1-mg/ml solution of a duplex oligonucleotide (GGTGTCCG⋅CGGACACC) in 10 mM Mops/100 mM NaCl (pH 7). This reaction was allowed to proceed for 1.5 hr at ambient temperature. In reactions of calf thymus DNA with either malondialdehyde or β-benzoyloxyacrolein, DNA (0.35 mg/ml) was treated with the compounds at 37°C for 5 hr in 25 mM sodium phosphate (pH 7.4).
Detection and Quantitation of M1G Adduct.
Following enzymatic hydrolysis of DNA samples with DNase I, nuclease P1, and alkaline phosphatase (18), the M1G adduct was quantified by gas chromatography/electron capture/negative chemical ionization/mass spectrometry preceded by an immunoaffinity purification step as described in detail elsewhere (18).
RESULTS
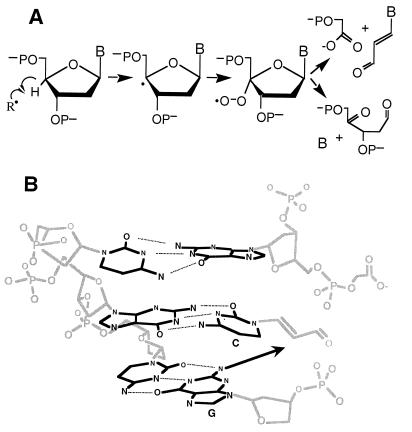
We first tested the hypothesis that chemicals causing formation of base propenals would also produce M1G. A base propenal is one of the products arising from radical-mediated abstraction of the 4′-hydrogen atom of deoxyribose. Following abstraction, the damage chemistry partitions to form either of two sets of products (Fig. 2): a strand break consisting of 3′-phosphoglycolate- and 5′-phosphate-ended DNA fragments and base propenal; or a ketoaldehyde abasic site and free base (15). Calicheamicin and bleomycin have been shown to cause 4′-hydrogen atom abstraction (15, 19, 20). Calicheamicin is an enediyne antibiotic that, when activated by thiols, forms a minor groove-specific diradical species that abstracts deoxyribose hydrogen atoms (15, 20). The resulting damage consists of bistranded lesions with 4′ chemistry on one strand and 5′ chemistry on the other (21). The iron-chelating antibiotic bleomycin produces both single-and double-strand DNA lesions caused only by 4′-hydrogen atom abstraction (15, 19).
Figure 2.
Formation of base propenal following radical-mediated 4′-hydrogen atom abstraction (15). (A) The pathway leading to base propenal is oxygen dependent for both calicheamicin and bleomycin, while the oxidized abasic site produced by bleomycin is oxygen independent (33). (B) A model for the formation of M1G in DNA by reaction of base propenal (cytidine) with a neighboring guanine.
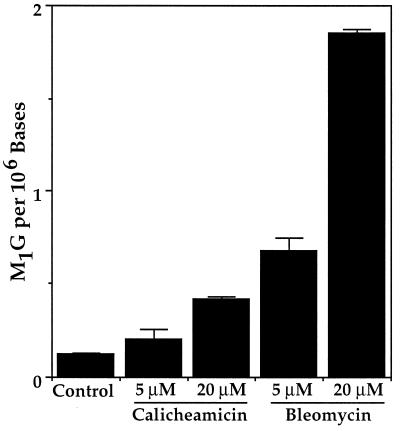
As shown in Fig. 3, treatment of DNA with calicheamicin and bleomycin caused a concentration-dependent formation of M1G. On a molar basis, bleomycin was three- to fourfold more efficient at causing formation of M1G than calicheamicin (1 × 1015 vs. 3 × 1014 M1G/mol, respectively). As discussed shortly, this difference may be due to limited redox cycling by the Fe(II)–bleomycin complex (calicheamicin is capable of only a single damage reaction), the reactivity of individual base propenals, or the proximity of base propenal formation to neighboring guanines.
Figure 3.
Formation of M1G in DNA treated with calicheamicin and bleomycin. Calf thymus DNA was treated with the indicated concentrations of these radical-mediated DNA cleavers and M1G was quantified as described in Materials and Methods. Error bars represent deviation about the mean for duplicate determinations.
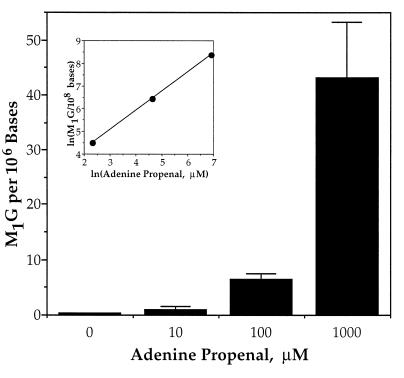
To establish that base propenal was responsible for the formation of M1G, we treated a duplex deoxyoligonucleotide with adenine propenal. As shown in Fig. 4, the base propenal caused a concentration-dependent formation of M1G. The inset in Fig. 4 attests to the linearity of the dose-response relationship.
Figure 4.
Formation of M1G in DNA treated with adenine propenal. Oligonucleotide DNA was treated with the indicated concentrations of adenine propenal and M1G was quantified as described in Materials and Methods. Error bars are deviation about the mean for triplicate determinations.
The greater reactivity of base propenal relative to malondialdehyde is demonstrated in Table 1. Of the three β-substituted acroleins tested, adenine propenal proved to be the most efficient at forming M1G. Both malondialdehyde and β-benzoyloxyacrolein required significantly higher concentrations and/or longer incubation times to achieve a similar level of adduction as adenine propenal. The M1G data for β-benzoyloxyacrolein, however, must be viewed as a conservative estimate given its instability in aqueous solution (12).
Table 1.
M1G formation by adenine propenal, β-benzoyloxyacrolein, and malondialdehyde
| Compound | mM | Incubation time, hr | M1G/106 bases |
|---|---|---|---|
| Adenine propenal | 0.1 | 1.5 | 6.5∗ |
| β-Benzoyloxyacrolein | 5 | 1.5 | 9.3† |
| Malondialdehyde | 5 | 28 | 0.5† |
Data from Fig. 3; 1 mg/ml DNA in 10 mM Mops/100 mM NaCl (pH 7.4), ambient temperature.
DNA (0.35 mg/ml) in 25 mM sodium phosphate (pH 7.4) at 37°C.
DISCUSSION
We have demonstrated that base propenals are capable of forming the M1G adduct by a direct reaction of DNA with adenine propenal and indirectly as a consequence of DNA damage produced by oxidizing agents. Several lines of evidence suggest that base propenals derived from oxidative DNA damage will be a biologically important source of M1G. The first involves proximity to DNA. Although lipids may be a greater target for attack by free radicals than DNA (13), malondialdehyde produced in lipid membranes must diffuse to DNA, while base propenal is generated directly in DNA. An enhancement of M1G formation by proximity to the ultimate target may be compounded if the base propenal remains intercalated in DNA. This may explain the high level of M1G associated with bleomycin-induced DNA damage. Bleomycin causes damage mainly at the pyrimidine of G-Py sequences (22) with subsequent formation of thymine and cytosine propenals (23, 24). As illustrated in Fig. 2B, if the pyrimidine propenals retain their positions by base stacking and by hydrogen bonding to the opposing purine, then the proximity of the neighboring guanine may enhance the rate of formation of M1G.
A second important point is the higher reactivity of adenine propenal, and probably other base propenals, than malondialdehyde in the formation of M1G. Adenine propenal was predicted to be more reactive than malondialdehyde since the imine nitrogen of a DNA base should be a better leaving group than the enol hydroxyl group of malondialdehyde at neutral pH, which bears a full negative charge (pKa = 4.6) (9). Many oxidizing agents, such as radiation and endogenous Fenton chemistry, are capable of forming both malondialdehyde and base propenal in cells (reviewed in refs. 3 and 25). However, even in the presence of much higher levels of malondialdehyde, the more reactive base propenal may represent a significant source of M1G.
One possible example of the biological implications of base propenal-derived M1G lies in the mutational spectra observed with bleomycin and neocarzinostatin (26–28); the latter is an enediyne similar to calicheamicin that produces base propenals (15, 29). Although many of the mutations caused by these agents can be explained by the formation of apurinic/apyrimidinic lesions at sites of damage, a significant number of the mutations do not occur at known damage sites (26–28). These transition and transversion mutations are consistent with M1G produced by diffusible base propenal (11).
An important question that remains to be answered is the relative contributions of lipid peroxidation (malondialdehyde) and oxidative DNA damage (base propenal) to the cellular burden of M1G. The levels of M1G in several tissues are similar to the levels of 8-oxoG, an oxidative DNA damage product: rat liver, 5 M1G vs. 6 8-oxoG/107 bases (30, 31); and human pancreas, 32 M1G vs. 19 8-oxoG/108 bases (F. F. Kadlubar et al., submitted for publication). Furthermore, of four types of base damage examined in a study of human pancreas (M1G, 8-oxoG, 1,N6-ethenoadenine, and 3,N4-ethenocytosine), only M1G and 8-oxoG showed a significant correlation (F. F. Kadlubar et al., submitted for publication). However, it has also been observed that exposure of rats to carbon tetrachloride caused an increase in the quantity of M1G in the liver (5) but did not affect the level of 8-oxoG (32). Though this problem awaits definitive studies, the relative contributions of malondialdehyde and base propenal to the quantity of M1G in a cell is likely to be tissue and oxidant specific.
In conclusion, we have demonstrated that base propenal, a product of oxidative DNA damage, reacts with DNA to form M1G adducts. In view of these results, it is likely that other electrophilic deoxyribose degradation products will also be genotoxic, such as the formylphosphate residues associated with 5′-hydrogen atom abstraction (15). Given the continuous production of oxidative DNA lesions in cells and the adventitious location of DNA-generated electrophiles, the base propenal-induced formation of M1G may represent a significant mutagenic burden in vivo.
Acknowledgments
We are grateful to Punam Mathur for her excellent technical assistance, Professor Francis Johnson for his guidance and advice in the procurement and use of adenine propenal, and Professor Steven Tannenbaum for critical reading of the manuscript. This work was supported by National Institutes of Health Grants CA64524 (to P.C.D.) and CA47479 (to L.J.M.) and the Samuel A. Goldblith Professorship (to P.C.D.).
ABBREVIATION
- Adenine propenal
9-(3-oxoprop1-enyl)adenine
References
- 1.Ames B N, Gold L S, Willett W C. Proc Natl Acad Sci USA. 1995;92:5258–5265. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ames B N, Shigenaga M K, Hagen T M. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Sonntag C. The Chemical Basis of Radiation Biology. New York: Taylor & Francis; 1987. [Google Scholar]
- 4.Breen A P, Murphy J A. Free Rad Biol Med. 1995;18:1033–1077. doi: 10.1016/0891-5849(94)00209-3. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhary A K, Nokubo M, Reddy G R, Yeola S N, Morrow J D, Blair I A, Marnett L J. Science. 1994;265:1580–1582. doi: 10.1126/science.8079172. [DOI] [PubMed] [Google Scholar]
- 6.Lindahl T. Nature (London) 1993;362:709–714. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 7.Ames B N, Gold L S. Mutat Res. 1991;250:3–16. doi: 10.1016/0027-5107(91)90157-j. [DOI] [PubMed] [Google Scholar]
- 8.Mukai F H, Goldstein B D. Science. 1976;191:868–869. doi: 10.1126/science.766187. [DOI] [PubMed] [Google Scholar]
- 9.Basu A K, O’Hara S M, Valladier P, Stone K, Mols O, Marnett L J. Chem Res Toxicol. 1988;1:53–59. doi: 10.1021/tx00001a010. [DOI] [PubMed] [Google Scholar]
- 10.Fink S P, Reddy G R, Marnett L V. Proc Natl Acad Sci USA. 1997;94:8652–8657. doi: 10.1073/pnas.94.16.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benamira M, Johnson K, Chaudhary A, Bruner K, Tibbetts C, Marnett L J. Carcinogenesis. 1995;16:93–99. doi: 10.1093/carcin/16.1.93. [DOI] [PubMed] [Google Scholar]
- 12.Basu A K, Marnett L J. Cancer Res. 1984;44:2848–2854. [PubMed] [Google Scholar]
- 13.Dix T A, Aikens J. Chem Res Toxicol. 1993;6:2–18. doi: 10.1021/tx00031a001. [DOI] [PubMed] [Google Scholar]
- 14.Price M A, Tullius T D. Methods Enzymol. 1992;212:194–218. doi: 10.1016/0076-6879(92)12013-g. [DOI] [PubMed] [Google Scholar]
- 15.Dedon P C, Goldberg I H. Chem Res Toxicol. 1992;5:311–332. doi: 10.1021/tx00027a001. [DOI] [PubMed] [Google Scholar]
- 16.Grollman A P, Takeshita M, Pillai K M R, Johnson F. Cancer Res. 1985;45:1127–1131. [PubMed] [Google Scholar]
- 17.Grollman A P. Dev Oncol. 1988;53:79–90. [Google Scholar]
- 18.Rouzer C A, Chaudhary A K, Nokubo M, Ferguson D M, Reddy G R, Blair I A, Marnett L J. Chem Res Toxicol. 1997;10:181–188. doi: 10.1021/tx9601216. [DOI] [PubMed] [Google Scholar]
- 19.Stubbe J, Kozarich J W. Chem Rev. 1987;87:1107–1136. [Google Scholar]
- 20.Lee M D, Ellestad G A, Borders D B. Acc Chem Res. 1991;24:235–243. [Google Scholar]
- 21.Hangeland J J, De Voss J J, Heath J A, Townsend C A, Ding W-d, Ashcroft J S, Ellestad G A. J Am Chem Soc. 1992;114:9200–9202. [Google Scholar]
- 22.Takeshita M, Kappen L S, Grollman A P, Eisenberg M, Goldberg I H. Biochemistry. 1981;20:7599–7506. doi: 10.1021/bi00529a039. [DOI] [PubMed] [Google Scholar]
- 23.Burger R M, Berkowitz A R, Peisach J, Horwitz S B. J Biol Chem. 1980;255:11832–11838. [PubMed] [Google Scholar]
- 24.Sugiyama H, Xu C, Murugesan N, Hecht S M. J Am Chem Soc. 1985;107:4104–4105. [Google Scholar]
- 25.Halliwell B, Gutteridge J M C. Free Radicals in Biology and Medicine. Oxford: Clarendon; 1989. [Google Scholar]
- 26.Povirk L F, Goldberg I H. Biochimie. 1987;69:815–823. doi: 10.1016/0300-9084(87)90208-2. [DOI] [PubMed] [Google Scholar]
- 27.Povirk L F. Mutat Res. 1987;180:1–9. doi: 10.1016/0027-5107(87)90061-3. [DOI] [PubMed] [Google Scholar]
- 28.Povirk L F, Goldberg I H. Nucleic Acids Res. 1986;14:1417–1426. doi: 10.1093/nar/14.3.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldberg I H. Acc Chem Res. 1991;24:191–198. [Google Scholar]
- 30.Nakae D, Mizumoto Y, Kobayashi E, Noguchi O, Konishi Y. Cancer Lett. 1995;97:233–239. doi: 10.1016/0304-3835(95)03980-b. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhary A K, Nokubo M, Marnett L J, Blair I A. Biol Mass Spectrom. 1994;23:457–464. doi: 10.1002/bms.1200230802. [DOI] [PubMed] [Google Scholar]
- 32.de Zwart L L, Hermanns R C, Meerman J H, Commandeur J N, Salemink P J, Vermeulen N P. Toxicol Appl Pharmacol. 1998;148:71–82. doi: 10.1006/taap.1997.8310. [DOI] [PubMed] [Google Scholar]
- 33.Babow L E, McGall G H, Stubbe J, Kozarich J W. J Am Chem Soc. 1990;112:3203–3208. [Google Scholar]