Abstract
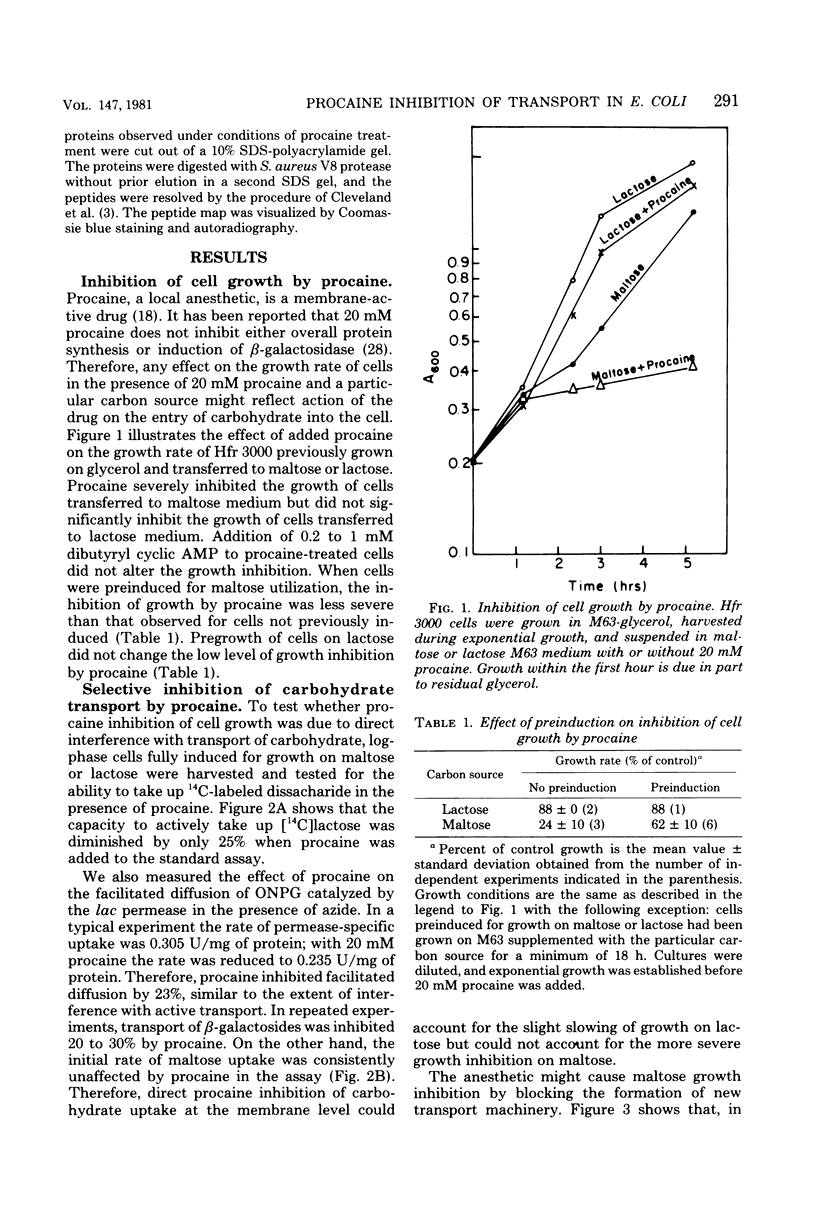
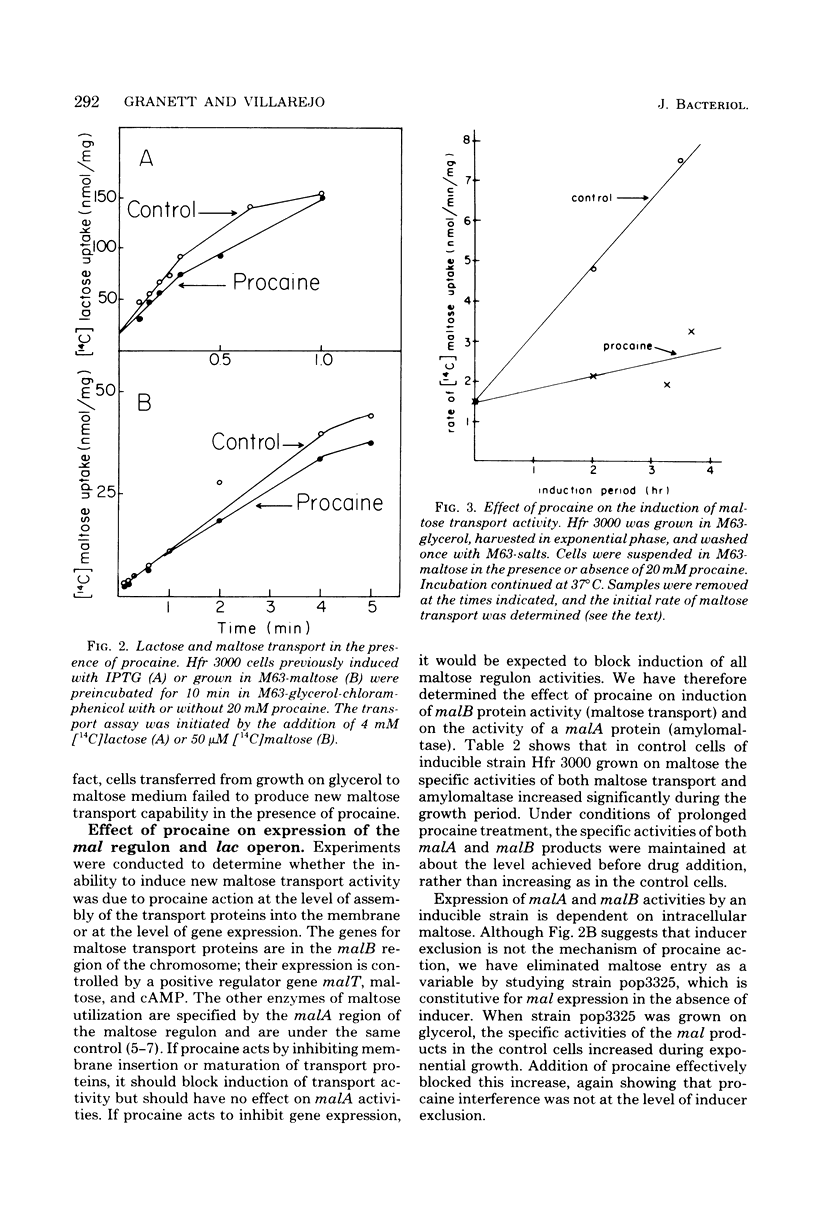
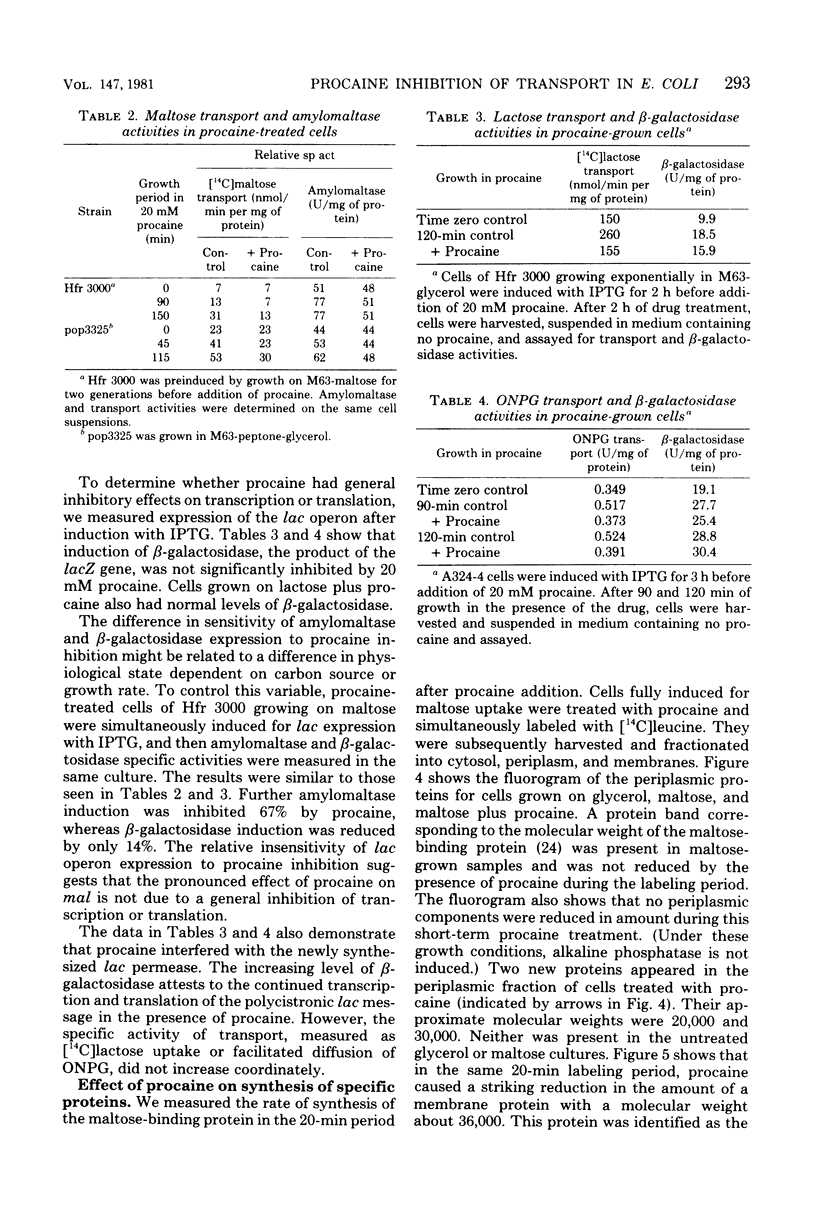
Maltose and lactose transport systems have been used to investigate the action of procaine on insertion and activity of membrane proteins and translocation of exported proteins in Escherichia coli. Procaine mildly inhibited growth on lactose. The level of inhibition was consistent with the small reduction observed in active and facilitated transport functions of the lac permease. However, procaine caused a severe reduction of growth rate on maltose, as well as an inhibition of induction of maltose regulon activities. In both constitutive and inducible strains, the synthesis of both maltose transport activity (malB operon) and amylomaltase activity (malA operon) was inhibited. Coordinate inhibition of soluble and membrane products was not observed with the lac operon. beta-Galactosidase synthesis proceeded normally during growth on procaine, whereas, the appearance of new transport activity was reduced. Regardless of carbon source, procaine specifically inhibited the appearance of ompF protein in the membrane fraction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson S. A. Local anesthetics block induction of the Pseudomonas alk regulon. J Bacteriol. 1979 Dec;140(3):1123–1125. doi: 10.1128/jb.140.3.1123-1125.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cronan J. E., Jr, Gelmann E. P. Physical properties of membrane lipids: biological relevance and regulation. Bacteriol Rev. 1975 Sep;39(3):232–256. doi: 10.1128/br.39.3.232-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debarbouille M., Schwartz M. The use of gene fusions to study the expression of malT the positive regulator gene of the maltose regulon. J Mol Biol. 1979 Aug 15;132(3):521–534. doi: 10.1016/0022-2836(79)90273-0. [DOI] [PubMed] [Google Scholar]
- Débarbouillé M., Schwartz M. Mutants which make more malT product, the activator of the maltose regulon in Escherichia coli. Mol Gen Genet. 1980;178(3):589–595. doi: 10.1007/BF00337865. [DOI] [PubMed] [Google Scholar]
- Débarbouillé M., Shuman H. A., Silhavy T. J., Schwartz M. Dominant constitutive mutations in malT, the positive regulator gene of the maltose regulon in Escherichia coli. J Mol Biol. 1978 Sep 15;124(2):359–371. doi: 10.1016/0022-2836(78)90304-2. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gayda R. C., Henderson G. W., Markovitz A. Neuroactive drugs inhibit trypsin and outer membrane protein processing in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 May;76(5):2138–2142. doi: 10.1073/pnas.76.5.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERZENBERG L. A. Studies on the induction of beta-galactosidase in a cryptic strain of Escherichia coli. Biochim Biophys Acta. 1959 Feb;31(2):525–538. doi: 10.1016/0006-3002(59)90029-0. [DOI] [PubMed] [Google Scholar]
- HORIUCHI T., TOMIZAWA J. I., NOVICK A. Isolation and properties of bacteria capable of high rates of beta-galactosidase synthesis. Biochim Biophys Acta. 1962 Jan 22;55:152–163. doi: 10.1016/0006-3002(62)90941-1. [DOI] [PubMed] [Google Scholar]
- Halegoua S., Inouye M. Translocation and assembly of outer membrance proteins of Escherichia coli. Selective accumulation of precursors and novel assembly intermediates caused by phenethyl alcohol. J Mol Biol. 1979 May 5;130(1):39–61. doi: 10.1016/0022-2836(79)90551-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazdunski C., Baty D., Pagès J. M. Procaine, a local anesthetic interacting with the cell membrane, inhibits the processing of precursor forms of periplasmic proteins in Escherichia coli. Eur J Biochem. 1979 May 2;96(1):49–57. doi: 10.1111/j.1432-1033.1979.tb13012.x. [DOI] [PubMed] [Google Scholar]
- Maloney P. C., Wilson T. H. Quantitative aspects of active transport by the lactose transport system of Escherichia coli. Biochim Biophys Acta. 1973 Dec 13;330(2):196–205. doi: 10.1016/0005-2736(73)90225-3. [DOI] [PubMed] [Google Scholar]
- Palmer T. N., Ryman B. E., Whelan W. J. The action pattern of amylomaltase from Escherichia coli. Eur J Biochem. 1976 Oct 1;69(1):105–115. doi: 10.1111/j.1432-1033.1976.tb10863.x. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D. Studies on the mechanism of action of local anesthetics with phospholipid model membranes. Biochim Biophys Acta. 1972 Apr 18;265(2):169–186. doi: 10.1016/0304-4157(72)90001-9. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Conrard D. J., Schnaitman C. A., Gregg T. I. In vivo effects of local anesthetics on the production of major outer membrane proteins by Escherichia coli. Biochim Biophys Acta. 1980 Jun 20;599(1):1–12. doi: 10.1016/0005-2736(80)90051-6. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Synthesis of exported proteins by membrane-bound polysomes from Escherichia coli. Eur J Biochem. 1977 May 2;75(1):43–53. doi: 10.1111/j.1432-1033.1977.tb11502.x. [DOI] [PubMed] [Google Scholar]
- Richards C. D., Martin K., Gregory S., Keightley C. A., Hesketh T. R., Smith G. A., Warren G. B., Metcalfe J. C. Degenerate perturbations of protein structure as the mechanism of anaesthetic action. Nature. 1978 Dec 21;276(5690):775–779. doi: 10.1038/276775a0. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Silva M. T., Sousa J. C., Polónia J. J., Macedo P. M. Effects of local anesthetics on bacterial cells. J Bacteriol. 1979 Jan;137(1):461–468. doi: 10.1128/jb.137.1.461-468.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmelcman S., Schwartz M., Silhavy T. J., Boos W. Maltose transport in Escherichia coli K12. A comparison of transport kinetics in wild-type and lambda-resistant mutants as measured by fluorescence quenching. Eur J Biochem. 1976 May 17;65(1):13–19. doi: 10.1111/j.1432-1033.1976.tb10383.x. [DOI] [PubMed] [Google Scholar]
- Tribhuwan R. C., Pradhan D. S. Induction of alkaline phosphatase in Escherichia coli: effect of procaine hydrochloride. J Bacteriol. 1977 Aug;131(2):431–437. doi: 10.1128/jb.131.2.431-437.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varenne S., Piovant M., Pagès J. M., Lazdunski C. Evidence for synthesis of alkaline phosphatase on membrane-bound polysomes in Escherichia coli. Eur J Biochem. 1978 May 16;86(2):603–606. doi: 10.1111/j.1432-1033.1978.tb12344.x. [DOI] [PubMed] [Google Scholar]
- Villarejo M. Evidence for two lac Y gene derived protein products in the E. coli membrane. Biochem Biophys Res Commun. 1980 Mar 13;93(1):16–23. doi: 10.1016/s0006-291x(80)80239-7. [DOI] [PubMed] [Google Scholar]
- Villarejo M., Ping C. Localization of the lactose permease protein(s) in the E. coli envelope. Biochem Biophys Res Commun. 1978 Jun 14;82(3):935–942. doi: 10.1016/0006-291x(78)90873-2. [DOI] [PubMed] [Google Scholar]
- Wandersman C., Moreno F., Schwartz M. Pleiotropic mutations rendering Escherichia coli K-12 resistant to bacteriophage TP1. J Bacteriol. 1980 Sep;143(3):1374–1383. doi: 10.1128/jb.143.3.1374-1383.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]