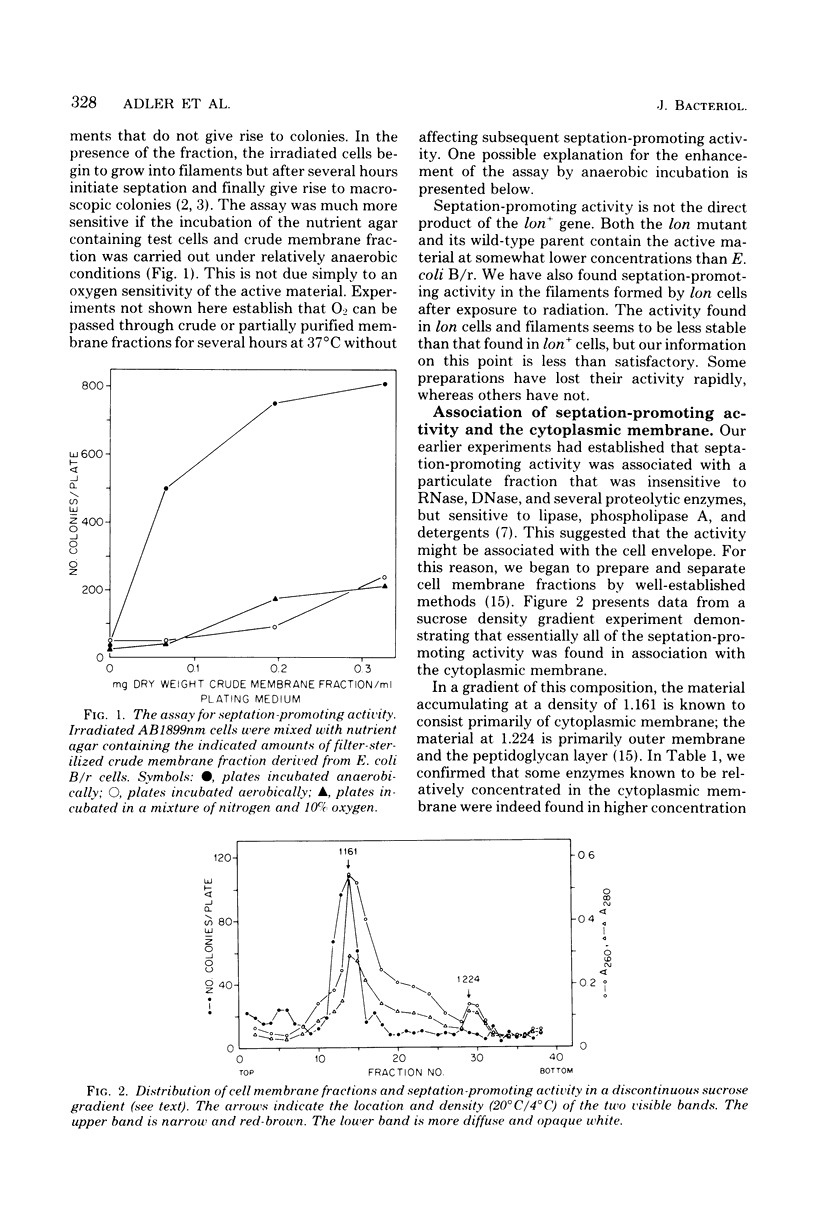
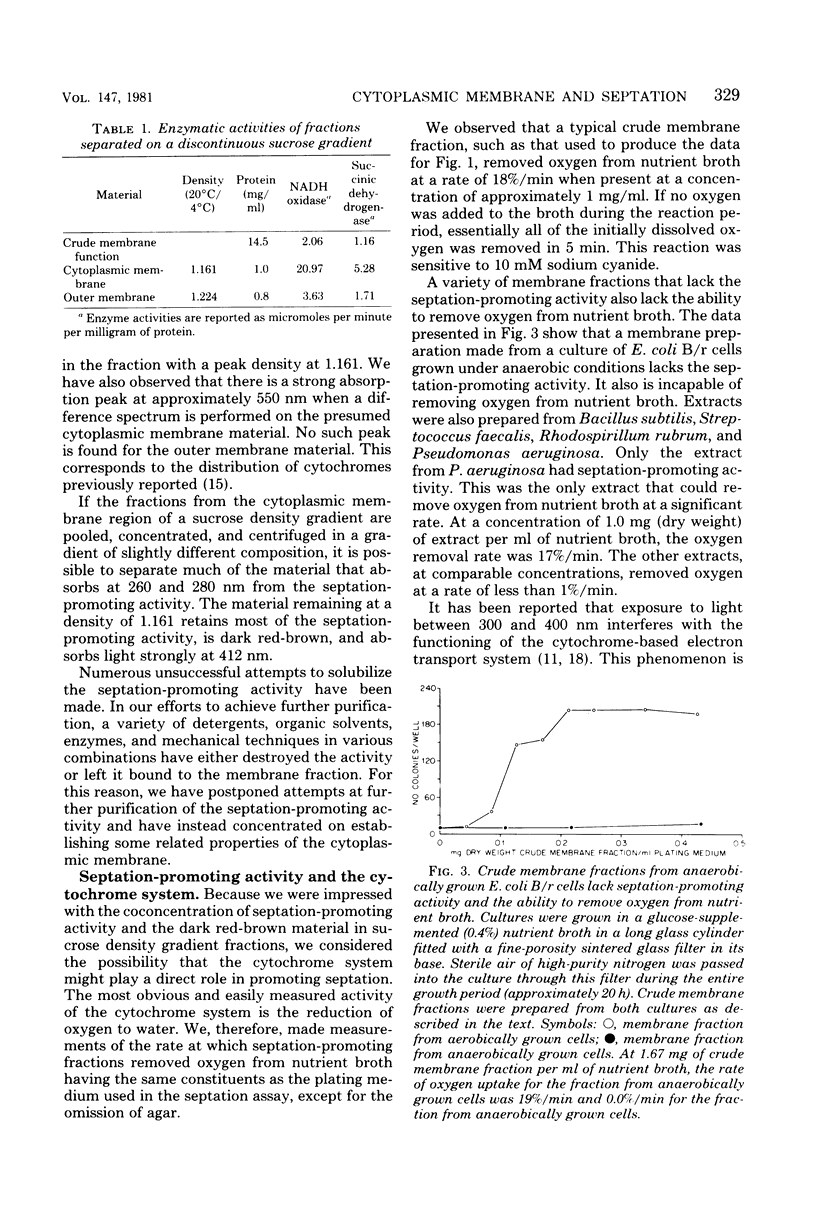
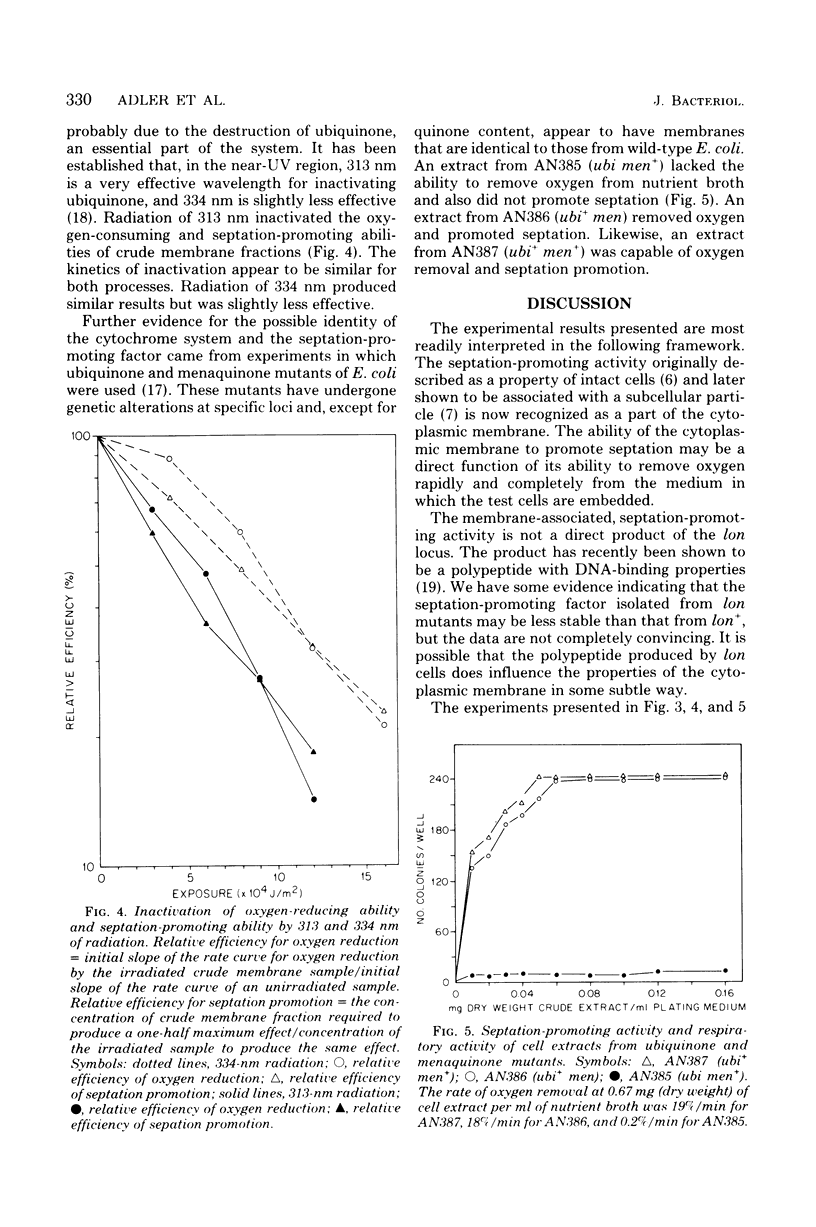
Abstract
A particulate fraction derived from bacterial cells stimulates septation in irradiated Escherichia coli lon mutants when added to postirradiation plating media. It was established that the particles are derived from the cytoplasmic membrane and that they have been partially purified by sucrose density gradient centrifugation. These particles also contain the cytochrome-based respiratory activity of the cell. A variety of experiments established a correlation between the septation-promoting activity of the particles and their ability to remove oxygen from the postirradiation plating medium. It was suggested that the efficient removal of oxygen from the medium allowed the lon cells to repair radiation-induced damage to the septation mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLER H. I., HARDIGREE A. A. POSTIRRADIATION GROWTH, DIVISION, AND RECOVERY IN BACTERIA. Radiat Res. 1965 May;25:92–102. [PubMed] [Google Scholar]
- ADLER H. I., HASKINS S. D. Heterogeneity of cultures of Escherichia coli B/r. Nature. 1960 Oct 15;188:249–251. doi: 10.1038/188249a0. [DOI] [PubMed] [Google Scholar]
- ALPER T., GILLIES N. E. The relationship between growth and survival after irradiation of Escherichia coli strain B and two resistant mutants. J Gen Microbiol. 1960 Feb;22:113–128. doi: 10.1099/00221287-22-1-113. [DOI] [PubMed] [Google Scholar]
- Adler H. I., Fisher W. D., Hardigree A. A., Stapleton G. E. Repair of radiation-induced damage to the cell division mechanism of Escherichia coli. J Bacteriol. 1966 Feb;91(2):737–742. doi: 10.1128/jb.91.2.737-742.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELAPORTE B. La restauration par voisinage chez des bactéries irradiées par des rayons X. Ann Inst Pasteur (Paris) 1956 Nov;91(5):727–735. [PubMed] [Google Scholar]
- Fisher W. D., Adler H. I., Shull F. W., Jr, Cohen A. Properties of a cell fraction that repairs damage to the cell division mechanism of Escherichia coli. J Bacteriol. 1969 Feb;97(2):500–505. doi: 10.1128/jb.97.2.500-505.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG J. A LOCUS FOR RADIATION RESISTANCE IN ESCHERICHIA COLI. Genetics. 1964 May;49:771–778. doi: 10.1093/genetics/49.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKINS B., ALPER T. Anaerobic growth as a factor influencing radiosensitivity. J Gen Microbiol. 1963 Feb;30:307–315. doi: 10.1099/00221287-30-2-307. [DOI] [PubMed] [Google Scholar]
- KASHKET E. R., BRODIE A. F. Oxidative phosphorylation in fractionated bacterial systems. X. Different roles for the natural quinones of Escherichia coli W in oxidative metabolism. J Biol Chem. 1963 Jul;238:2564–2570. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. J., Young I. G. Role of quinones in electron transport to oxygen and nitrate in Escherichia coli. Studies with a ubiA- menA- double quinone mutant. Biochim Biophys Acta. 1977 Jul 7;461(1):84–100. doi: 10.1016/0005-2728(77)90071-8. [DOI] [PubMed] [Google Scholar]
- Werbin H., Lakchaura B. D., Jagger J. Near-ultraviolet modification of Escherichia coli B ubiquinone in vivo and in vitro. Photochem Photobiol. 1974 May;19(5):321–328. doi: 10.1111/j.1751-1097.1974.tb06519.x. [DOI] [PubMed] [Google Scholar]
- Zehnbauer B. A., Markovitz A. Cloning of gene lon (capR) of Escherichia coli K-12 and identification of polypeptides specified by the cloned deoxyribonucleic acid fragment. J Bacteriol. 1980 Aug;143(2):852–863. doi: 10.1128/jb.143.2.852-863.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


